Henry David Thoreau wrote, “If the day and night are such that you greet them with joy and life emits a fragrance like flowers and sweet-scented herbs—that is your success. All nature is your congratulation.” Generations ago, Thoreau had written these thoughts about the importance of floral fragrances and sweet-scented herbs. This concept maintains its importance in today’s dot-com world. In fact, it has become more meaningful as human beings become more dependent on machines and lose touch with their appreciation of nature. Consequently, cosmetics incorporating fragrances act as a boon for mankind. In one respect, fragrance is highly important in the development of products, from chewing gum and cosmetic applications to preventive care.
On the other hand, fragrance also could be considered a nonfunctional ingredient that is more expensive per pound than other ingredients, as well as being replaceable without affecting the performance of the product. It therefore could be considered efficient manufacturing to cut fragrance costs to the minimum as long as the formula is compatible with reasonable consumer acceptance.
Needless to say, the two viewpoints—fragrance as an ornament versus fragrance as a communication channel—often lead to different fragrance selections.
Today, antiperspirants and deodorants represent one of the largest personal care product categories. More than 90% of the US population currently uses such products daily, and a similar trend will follow in other regions. In antiperspirants and deodorants, active ingredients or product formulations can alter the perfume oils or individual odorants in the aqueous alcoholic solutions. For this reason, it is important to conduct comprehensive stability tests with products before their large-scale manufacture and distribution.
Deodorants
Deodorants contain topical germicides such as quaternary ammonium compounds, parabens and triclosan. These compounds significantly reduce the number of skin bacteria that cause body odor through bacterial decomposition of perspiration residue.
Deodorants are designed to reduce axillary odor. Since this is considered a nontherapeutic purpose and the body is not considered to be altered by their use, they are classified as cosmetics.
Fragrance suppliers generally carry out extensive stability tests on individual aroma materials before a fragrance is created.1 The interaction of the actives with fragrance materials is evaluated on the basis of odor deterioration and discoloration. Perfumers consider these results in selecting materials for their creative work.
Since a fragrance is a complex mixture of aromatic materials of natural and/or synthetic origin, it is difficult to ensure that all ingredients present will be stable and free from degradative changes induced by the formulation of the product.
Approximately 1–2% fragrance is required to cover the base odor and release desirable top notes during use. The most common fragrance types are: spicy, oriental, green, woody, baby powder, mint, citrus aldehyde, floral, green herbal and lime. A successful fragrance must coordinate with the product’s attributes. Its initial impact, continuing impressions, performance and stability are crucial in ensuring a harmonious product. Therefore, the perfume must contain a bouquet of carefully constructed notes to signal the excellence of its functional performance at all stages.2 Top notes are used to confirm the proclamations of the media and make initial sales, thereafter acting as recognition signals; middle notes establish and reinforce perceived functional excellence, and base notes are for sufficient substantivity.
The whole of the above sequence fragrance performance is made possible by considering the volatility, solubility and substantivity of the ingredients in the perfume as much as its fragrance properties in the product under shelf and in-use conditions; for example, underarm deodorants that incorporate actives such as alcohol, triclosan and propylene glycol with fragrances constituted of aldehydes, alcohols, ketones and ethers.
Product profile: Deodorants are defined as cosmetic products that deodorize perspiration without examining its flow to any appreciable extent.3
Deodorants are commonly packaged in many forms: stick solids, pads, dabber units, aerosols, roll-ons, pump sprays, squeeze creams, creams, stick creams and soaps.
Mechanism of action: The human axilla provides ideal ecological factors for bacterial growth.4 The rich supply of eccrine sweat glands that provide water, electrolytes and minerals; apocrine sweat glands that secrete a substance rich in protein and lipid; and sebaceous glands, which produce a mixture of lipids, contribute to the ecology of the axilla. The outermost region of the skin, the stratum corneum (SC), is a compartment that constantly is being shed and provides a rich source of amino acids necessary for bacterial growth.
Sweat glands deliver water to the surface and provide the critical moisture required for bacterial proliferation. In addition, sweat contains amino acids and minerals such as copper, iron, magnesium, zinc and calcium, which are important for bacterial growth and metabolism as well as toxin production by pathogenic bacteria. The presence of large amounts of water and the rich supply of proteins, lipids and minerals, coupled with the anatomy of the axilla, which produces a semi-occluded environment that minimizes evaporation of water, results in an ideal ecosystem for bacterial growth.
Axillary odor is a mixture of many notes with the dominating notes identified as isovaleric acid and 5-androst-16-en-3-one and 5-androst-16-en-3-ol. The alcohol has a musky odor that is not unpleasant; while the ketone confers a disagreeable odor that is often described as stale urine. Isovaleric acid has a “sweat” odor.
Apocrine secretion is odorless but does contain significant amounts of cholesterol and two sulfated steroids—androsterone sulfate and dehydroepiandrosterone sulfate. The cholesterol, other steroids and proteinaceous substances present in apocrine secretion provide an ecosystem for bacterial growth and odor development.
Other substances in the axilla originating from sebaceous and eccrine glands also may contribute to the total odor profile of the axilla. Sebum intermingles with apocrine secretion in the infundibulum of the follicle and contains approximately 10% squalene, a material that is used as a fixative in fragrances and may be important in prolonging axillary odor.
The composition of sweat may actually vary within a rather wide range.5 It depends on diet, muscular activity during perspiration and many other factors.
Fresh perspiration has a very faint and not unpleasant odor. The characteristic odor of perspiration develops only after bacteria have acted on apocrine sweat. Eccrine glands secrete a clear acid (pH 4–6.8), which is attributed to the presence of fatty acids (C-C10, butyric to capric acid), NH3 and amines, mercaptans and other sulfurous substances derived from the degradation of sulfurous proteins. Action of bacteria is really of great importance. A mixture of eccrine and apocrine sweat develops an unpleasant odor if it is left standing for sometime.
Deodorant Ingredients
The antimicrobial efficacy of ingredients used in modern deodorant fragrances is described below.6
Triclosan: Triclosan is effective against Gram-positive and Gram-negative bacteria. Triclosan levels for underarm deodorants are somewhat lower because these products are left on the skin at a range of 0.03–0.3%.7 The primary site of action of triclosan is the cell membrane.8 At low bacteriostatic concentrations, this action on the membrane interfaces with the uptake of amino acids, uracil or other nutrients from the medium. At higher bactericidal concentrations, the membrane lesions lead to the leakage of cellular content and the death of the cell.
Meincke et al.9 speculated that the uptake of triclosan by both cell walls and whole cells is due to the hydrophobic and lipophilic nature of the antibacterial agent and its greater solubility in the cell components than in the surrounding water-rich environment. Resistance of such microorganisms as Pseudomonas aeruginosa may be due to higher lipid content in the cell wall; a higher lipid content of the cell wall would tend to adsorb more triclosan thus hindering the access of the drug to its proposed site of action—the cytoplasmic membrane.
Propylene glycol: As a surfactant or wetting agent and solvent, propylene glycol actually is the active component in antifreeze. Industry in general uses it to break down protein and cellular structures and it is found in most forms of deodorants and food processing.
Personal fragrances: Most fragrances for personal use are presented as dilutions of perfume compounds in suitable solvents, as dispersions in specially formulated creams, or in stick form.10
Alcohol: In the formulation of cosmetics and toiletries, certain alcohols serve as both preservatives and solvents.11 As a chemical group, the alcohols possess many desirable features. They are bactericidal rather than bacteriostatic; they are colorless, have a cleansing action; and in the case of lower aliphatic alcohols, they evaporate readily. Compared with other aliphatic alcohols, ethanol has less odor and toxicity, giving it wide acceptance as a solvent and preservative.
Mechanisms of Action
Several mechanisms of action have been offered to explain the effects that deodorant fragrances have on malodor.
Odor modification: Odor modification involves masking or changing a malodor to a more pleasant character and/or reducing the odor intensity to a more acceptable level, i.e., counteraction.
Odor desensitization: Some materials may temporarily deactivate nasal sensory receptors and thus act as a deodorant. An example of this effect was found by Torii and Egma, who prepared isolates from the Theaceae plant, presumed to contain flavanols, and found deodorant activity.12 This deodorant effectiveness may be due to a complex mechanism consisting of clathrating, addition and neutralization reactions with the active ingredient reactions in human beings, such as inhibition of olfactory receptors.
Odor absorption (by deo-fragrances): An odor absorption effect has been reported for some deodorant fragrances. As an example, one of the criteria for the deodorant components is that the component reduce the partial vapor pressure of morpholine by at least 10% more than that required by Raoult’s law.
Biochemical effects (by deo-fragrances): In addition to the absorption effects, fragrance materials show a significant capacity to inhibit the lipoxidase. A lipoxidase can catalyze the hydroperoxidation of polyunsaturated fatty acids and esters containing a cis, cis-1, 4-pentadiene moiety. Linoleic acid and esters of linoleic acid, which contain this pentadienyl group, are present in the epidermis. The hydroperoxides that could be generated by the action of lipoxidase on epidermal linoleates are known to decompose or undergo further oxidation to short- and medium-chain aldehydes, ketones and acids, most of which have strong, unpleasant odors.
Antimicrobial fragrances: Deo-safe fragrances not only reduce the perception of odor with odor-masking, but also stop odor development through antimicrobial action. In any case, the most successful applications of antimicrobial fragrances would probably be with products that are intended to be left on the skin.
Materials and Methods
A deodorant test sample (Formula 1) was prepared and divided into three parts.
One part was stored in a refrigerator at 10°C, another was stored at room temperature and the third was stored at 48°C in an oven, for 3 months. The deodorant samples were tested for pH, sensorial properties, skin sensitivity via patch test, intensity and substantivity. Further, the deodorant was analyzed by gas chromatography (GC) for changes in the individual perfumery material.
GC analysis: GC analysis was performed using a chromatographawith a flame ionization detector.
A 0.5 g perfumed deodorant stick was combined with 5 mL of ethanol and shaken well for 2 min before filtering. This filtrate was then collected for GC analysis. The deodorant samples at room temperature and at 48°C were tested by GC for stability of fragrance material. The analysis was performed under an inlet temperature of 220°C, and detector temperature of 250°C. The oven temperature began at 80°C and was increased by 15°C/min for a total of 10 min.
Hedonic scale: A measure of the degree of acceptance of a product may be obtained by the use of the hedonic scale (HS). Panelists were asked to rate their degree of like or dislike in terms that best described their feelings about the product. These terms were given numerical values to enable the results to be scored; a rating of 1 being “disliked completely,” to 7 as being “liked completely.”
Intensity Scale: An intensity scale also was used to score the strength of a product; a rating of 0 meaning “no intensity/no odor,” to a rating of 10 meaning “extremely strong odor.”
Substantivity: On a special paper strip, a sample of the perfumed deodorant stick was placed and the strip was left open in a ventilated room. The time of evaporation was noted at one-hour intervals. In a substantivity test, the reading shows the number of hours the fragrance remains in a product at given conditions; these readings are the average of readings obtained from experts on a panel.
Patch test: The deodorant stick was rubbed on the skin for 5 sec and observed for skin irritation. The score was taken as follows: no reaction was marked as erythema only, “+”; erythema with papules, “++”; papilovasicular, “+++”; and ulceration or necrosis, “++++.”
Determination of pH: A pH meter equipped with a glass electrode was used to test pH. The pH of the deodorant sample at room temperature was determined by taking 2 g of the test material in a 150-mL beaker and adding 98 mL of freshly boiled and cooled water. This combination was stirred well to make a thorough suspension and the pH was taken using a pH meter within 5 min of preparing the suspension. The pH electrode was dipped in a beaker containing the deodorant stick and the reading was noted.
Discussion Figure 3
Esters: Esters in alkaline medium and in the presence of water are dissociated and form salts and corresponding alcohol. This is a harmful reaction decrease in the sweetness of the odor profile.
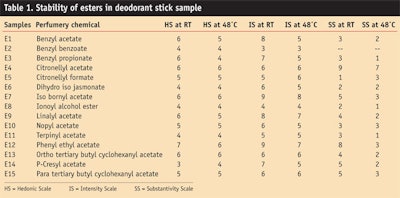
Table 1 and Figure 1, Figure 2 and Figure 3 show the stability study including the hedonic scale, intensity scale and substantivity of esters in the deodorant stick.
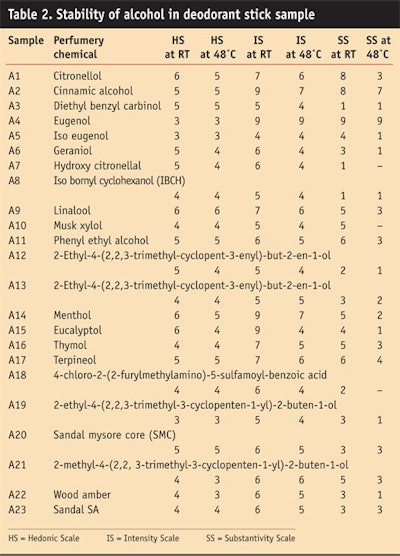
Alcohol: Table 2 and Figure 4, Figure 5 and Figure 6 show the stability study of alcohols, including the HS, intensity scale and substantivity, in a deodorant stick formulation.
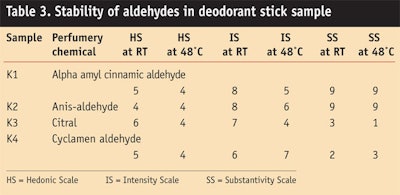
Aldehydes: Aldehydes such as aromatic terpene are used in deodorant stick formulations. They are very reactive and not stable in alkaline medium. In some cases, aldehyde reacts with ethanol present in the product and improves stability by forming hemiacetals. Table 3 and Figure 7, Figure 8 and Figure 9 show the stability study of aldehydes in deodorant stick formulations.
In general, alpha amyls cinnamic and cyclamen aldehyde give good stability and impart floral jasmine with green chararacter, which was liked by panelists.
Ketones: Table 4 and Figure 7, Figure 8 and Figure 9 show the stability study of ketones in deodorant formulations.
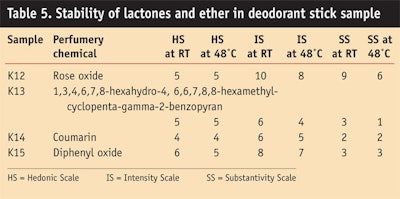
Lactones: Table 5 and Figure 7, Figure 8 and Figure 9 show the stability study of lactones in deodorant stick products.
Ether: Table 5 and Figure 7, Figure 8 and Figure 9 give the stability study of ethers in deodorant stick formulations. It was found that constituents of authentic diphenyl oxide material change to different constituents after 2 hr, after 3 months at room temperature, and at a high temperature of 48°C, but the odorant note remained pleasant. Diphenyl oxide at 1% in deodorant stick was good in imparting floral green with metallic notes and liked by panelists (HS 6); further, it had good intensity for masking base odor (8) and good substantivity (3).
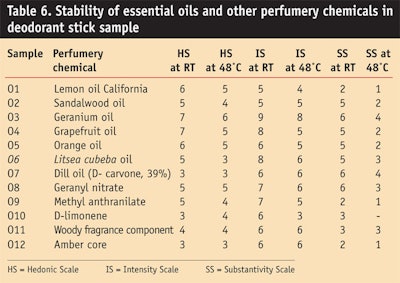
Essential oil: Table 6 and Figure 10, Figure 11 and Figure 12 show the stability study of essential oils and other chemicals in deodorant stick applications. In general, natural oils in an alkaline medium and in the presence of water exhibited discoloration.
Conclusion
In the described deodorant stick formula tested, the constituents provided a highly alkaline medium and affected the perfume material depending upon the time and temperature.
Aldehydes reacted with alcohol in the presence of alkali in the product, producing acetals. This conversion to acetal depended upon the time and temperature of the formula. As time passed and temperature changed, the yield of acetal varied. Hence, the retention time of fragrant materials changed. Aldehydes in a deodorant base gave good stability according to the odor profile at room temperature and at a high temperature of 48°C after 3 months. Alpha amyl cinnamic and cyclamen aldehyde gave good stability and imparted floral jasmine with green character, which generally was liked by panelists.
The alcohols were stable in alkaline medium. Cinnamic alcohol, geraniol and phenyl ethyl alcohol gave good stability in the sample formula and imparted floral rosy fragrance with a green note, which was liked by panelists. However, the menthol and eucalyptol imparted a camphraceous odor to the deodorant stick, which also was liked by the panel.
Esters such as benzyl acetate imparted a floral jasmine-like character to the sample formula, which was liked by the panel. Phenyl ethyl acetate gave a good hedonic scale and intensity rating after 3 months. It imparted a rosy floral, honey-like sweet character to the deodorant stick.
It was found that benzyl benzoate completely masked base odor of the deodorant stick and thus it can be used along with ethanol as a fixative. All the esters gave good hedonic scale ratings after 3 months at room temperature, as well as at high temperatures.
Ketones in the alkaline medium caused the hemiacetal formation. This is a beneficial reaction; however, further reaction is converted into acetal, which decreases the intensity of the perfume in a product, potentially rendering it odorless. In the ketone camphor, 7-acetyl, 1,2,3,4,5,6,7,8-octahydro-1,1,6,7-tetramethyl naphthaleneb, hedione and beta ionone gave good stability and were liked by the panelists.
The nitrile compound geranyl nitrile showed good stability and was accepted after 3 months by the panel.
Lactones such as rose oxide imparted penetrating green rose-like odor in the sample formula. After 3 months it too was rated favorable on the hedonic scale by panelists.
Natural oils showed discoloration in the sample stick formulation but the geranium oil, Litsea cubeba oil, dill oil and amber core oil gave good stability and a favorable hedonic scale rating after 3 months at room temperature and at high temperature of 48°C.
Deodorant products require specialized fragrances. Before a final fragrance can be selected, extensive stability testing on individual perfumery chemicals and essential oils should be carried out. The hedonic scale, intensity scale, substantivity test, patch test, pH determination, and odor changes should be closely monitored during stability testing. This work will help formulators understand the behavior of individual perfumery chemicals and essential oils in deodorant base, which will assist in fragancing products.
Acknowledgments: The authors wish to thank M/S, Goldfield Fragrance and Flavour Ltd., Mumbai, India, for its generous gift of all the perfumery chemicals. The authors also thank Sudhir D. Mestri, PhD, Dabur India Ltd., Mumbai, for providing valuable guidance. The author, Chintaman T. Bondar, would also like to thank the International Congress of Essential Oil, Flavor & Fragrances (ICEOFF), New Delhi, for the Research Fellowship award.
References
1. SP Gimelli, Aroma Science England, Micelle Press 517–563 (2001)
2. N Geria, Fragrancing antiperspirants and deodorants, current practices, stability problems and future trends, Cosm &Toil 105 4 41–45 (1990)
3. B Wilkinson and RJ Moore, Harry’s Cosmeticology, 7th ed, Chemical Publishing, New York 124–139 (1982)
4. K Laden and CB Felger, Antiperspirants and Deodorants, Marcel Dekker Inc., New York and Basel 345–350 (1988)
5. JS Jellinek, The Use of Fragrance in Consumer Products, Wiley-Interscience Publications, New York (1975)
6. J Zhou et al, Content uniformity of triclosan in some commercial deodorant sticks, Cosm &Toil, 105 4 47–48 (1990)
7. Irgasan DP 300 Broad Spectrum Bacteriostat, Ciba-Gigey Corp, Greensboro, NC (1983)
8. J Regos and HR Hitz, Investigations on the mode of action of triclosan, a broad spectrum antimicrobial agent, Zentralbl Baktinal.Hyg.I.Abt Orig.A 226 390–401 (1974)
9. BE Meincke, RG Kronz and DL Lynch, Effect of Irgasan on bacterial growth and its adsorption into the cell wall, Microbios 28 133–147 (1980)
10. SP Gimelli, Aroma Science England, Micelle Press 159–170 (2001)
11. J Bandelin, Antibacterial and preservative properties of alcohols, Cosm &Toil 92 5 59–70 (1977)
12. US patent 4,501,730, K Torii and C Egma