Skin protection against ultraviolet (UV) radiation has become a necessity as the connection between serious skin conditions and a changed lifestyle has become more obvious in recent years.1 This has led formulators to use UV filters not only in sunscreens, but also in face care products. Modern sunscreen formulations with high sun protection factors (SPFs) often rely on a mixture of organic and inorganic UV filters. For UVB protection, titanium dioxide (TiO2)-based materials are an addition or alternative to organic UV filters as they efficiently help to prevent sunburn without the inherent drawbacks of organic UV filters, such as light degradation and in some cases, an unfavorable toxicological profile.2 Further, the German Federal Institute for Risk Assessment (BfR) has come to the conclusion that TiO2 UV filters do not penetrate the dermis and pose no risk for the consumer.3
Titanium Dioxide
When first introduced, TiO2-based UV absorbers had a tendency to impart a whitening effect when applied to the skin. This whitening is not surprising, given the fact that several million tons of TiO2 are used as a white pigment in coatings and plastics due to its very high refractive index: 2.493 for anatase and 2.616 for rutile.4 However, to meet the cosmetic industry’s requirement for sunscreen formulations that efficiently filter out UVB radiation but have a low whitening effect, TiO2 grades with optimized properties were developed as early as the 1970s. For instance, by decreasing the primary particle size of TiO2 pigments, their ability to scatter visible light was reduced while maintaining a high UV-scattering ability.
This effect can be explained by the scattering theory of Gustav Mie.5 Light scattering and light absorption are differently dependent on particle size. For light scattering, which is responsible for the strong white pigment effect of TiO2, an optimal primary particle size can be defined; in this case, close to half of the wavelength of visible light, normally between 200 nm and 300 nm.6 Note that for this calculation, 550 nm is used as the representative wavelength for visible light because the human eye is most sensitive to this wavelength. By downsizing the primary size of TiO2 particles, the whitening effect is reduced while maintaining their UV protection. Therefore, a suitable primary particle size for TiO2 UV filters typically is in the range of 15–50 nm.
A change in the regulation of cosmetic products in Europe has made sunscreen producers uncertain about the use of mineral UV filters. In the new EU Cosmetic Regulation EC 1223/2009 coming into effect in 2013, the term nanomaterials was defined for the first time in a regulatory context.7 According to this definition, if TiO2-based UV absorbers fall into the “nanomaterials” category, their presence in any cosmetic formulation must be indicated by the suffix “(nano)” on the INCI ingredient list of such products. TiO2, which would be indicated with this suffix, is positively listed in Annex VI as a product that can be used in cosmetic formulation. Nevertheless, to avoid possible negative reactions from consumers due to this “(nano)” labelling, a search for inorganic UV filters not requiring this label has begun.8
Currently a controversy between regulatory requirements, sunscreen performance and aesthetics is occurring. While it may be desirable to increase the primary particle size of TiO2-based UV absorbers from the regulatory standpoint, in order to prevent this “(nano)” labelling in the ingredient list, experience in formulating these products indicates that particle sizes below 100 nm are needed for the performance of these products. Therefore, the aim of this study was to investigate how the primary particle size influences the application properties of TiO2 as a UV absorber in sunscreen formulations (see Formula 1).
Materials and Methods
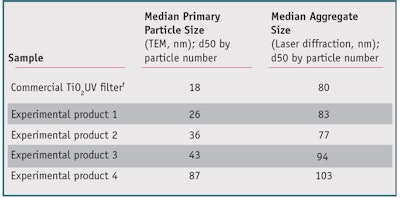
Experimental TiO2 UV absorbers: Experimental hydrophobic TiO2 UV absorbers were produced by high temperature hydrolysis of TiO2 in a hydrogen oxygen flame. In this process, initially water is formed by burning the hydrogen in the oxygen-rich gas mixture. This extremely hot water then hydrolyzes the gaseous titanium tetrachloride to form TiO2 and hydrogen chloride gas. The TiO2 particles formed are separated from gaseous byproducts. Determined by the reactor design and through precise control of process parameters, the key characteristics of these materials such as the primary particle size, aggregate size, shape and distribution could be tailored. The surface modification of such products was based on the reaction of the external TiO2 surface with suitable reagents, in this case trimethoxycaprylylsilane. By reaction of the trimethoxycaprylsilane with surface hydroxyl groups of the TiO2, strongly hydrophobic octyl groups are irreversibly anchored to the surface of the particles. The alcohol released by this reaction from the alkoxy silane is completely removed by heat treatment of the surface modified titanium dioxide powder. Key characteristics of the surface modification process such as type and homogeneity were carefully controlled by process conditions. Main characteristics of the products generated by this process are summarized in Table 1.
Product characterization: Transmission electron microscopy (TEM) images were obtained using a calibrated microscopea. Approximately 2 mg of the test substances were dispersed in chloroform using ultrasound for 3 min. The primary particle size was determined by selecting representative sections of calibrated TEM images and printing them on transparent films, which were then analyzedb. Approximately 2,500 particles were selected, measured and statistically evaluated.
The external sizes of the aggregates and agglomerates of the test substances in dispersion were determined by laser diffraction granulometry via an instrumentc utilizing a universal liquid module. To prepare test samples, 7.5 g of the powders were dispersed in 142.5 g C12–15 alkyl benzoated using a rotor stator instrument equipped with a mixere. From the resultant dispersion, 2–3 drops were diluted with additional C12–15 alkyl benzoate directly before measurement. The benchmark was commercial hydrophobic titanium dioxidef.
Application tests: The oil-in-water test formulation shown in Formula 1 included emulsifier and emollient combinations that are known to have good dispersing properties for different kinds of TiO2 powders. This formulation allowed for good comparison of several parameters of different TiO2 types.
Whitening: The ΔL* values of each formulation sample based on the test formulation were measured with a colorimeterg. The L* value represents the black and white axis of the L*a*b color space. Glass slides, painted black on only one side, were coated with a 20 μm film of the formulations. For each sample, three different points of the respective film were measured three times each. The results recorded were the average of these nine measurements.
UV protection (in vitro): Each test formulation sample was applied evenly at 1 mg/cm2 on a PMMA slideh. The prepared slides were then stored for levelling 30 min at 30°C. The measurements and subsequent analysis of the data were conducted with a transmittance analyzerj and its associated software per the Cosmetics Europe (former Colipa) Guideline.9 Note that due to the low sun protection factor expected from the test formula with 5% TiO2 as the only UV filter, a variation of the guideline for the application on the PMMA plates was performed—i.e., 2 μm roughness of PMMA plates instead of 6 μm, and 1 mg/cm2 instead of 1.3 mg/cm2—in order to produce reliable data with a low deviation for this range of in vitro SPF. Each sample was measured four times at three spots on the slide. Therefore, each result is the medium average of 12 measurements. A formulation with TiO2 as the only UV filter was used to obtain the influence of change in primary particle size on the SPF without interference from the other UV filters.
Results
Product characterization: TEM images of the experimental products as well as the commercial hydrophobic TiO2 produced by the same processf are shown in Figure 1. The images of all products show aggregates, which are composed of primary particles covalently bound to each other. No isolated primary particles are observed in the TEM pictures, although there are differences in the shape and size of the primary particles as well as the state of aggregation.
Table 1 lists the sizes of the primary particles of all investigated TiO2 UV absorbers as measured by TEM, as well as the size of the aggregates/agglomerates after dispersion in C12–15 alkyl benzoate as measured by laser diffraction. Based on the TEM images, the size of the primary particles is visible and can be analyzed statistically. The median primary particle size increased from the commercial product to experimental Product 4.
Since the products do not exist as isolated primary particles in dispersion, external particle sizes when dispersed in C12–15 alkyl benzoate, as a typical emollient for sunscreen formulations, also were measured by laser diffraction. As expected, the external particle size of the products observed by laser diffraction was much higher and did not change much from the commercial product to experimental Product 4. The catalytic activity of all materials investigated in this study was determined in a 10% dispersion of the respective TiO2 sample in diethylhexyl carbonatek with 2% L(+)-ascorbyl palmitate. L*a*b values were measured after 2 hr and 24 hr. For all samples, very little discoloration was observed, indicating only minor catalytic activity. Furthermore, the samples showed only small differences in catalytic activity. The primary particle size therefore did not influence the catalytic activity because the degree of surface modification was adjusted for every sample.
Whitening: The whitening of the test formulas became stronger as the primary particle size of the respective TiO2 became larger (see Figure 2). Materials with higher median primary particle size, e.g., experimental Products 3 and 4, showed an unacceptable, increasingly strong white color during and after application. This whitening also depends on proper dispersion of the titanium dioxide in a formulation. The small increase of aggregate sizes in Table 1 shows that the influence of the primary particle size is more important for the whitening effect.
In vitro SPF and critical wavelength (λC): While the whitening of an inorganic UV filter is an unwanted but hazard-free effect, its ability to protect against UV radiation is essential for performance. Figure 3shows the sun protection factors of the test formulations using the different TiO2 UV absorbers. The sun protection factors decreased with an increasing median primary particle size of the filters used.
Figure 4 summarizes the effect of primary particle size on both in vitro SPF as well as the in vitro critical wavelength, λC. The in vitro critical wavelength is defined as the wavelength where the area under the absorbance spectrum from 290 nm to λC represents 90% of the area of the complete absorbance spectrum from 290 nm to 400 nm. With increasing particle size, the experimental products show an increase in λC, which means the absorption shifts to longer wavelengths.
A change from smaller to bigger primary particle size would mean the formulator must compensate for the decrease of SPF by higher use levels of such filters to obtain the same sun protection factor in the final sunscreen formulation. Since the absorbance spectrum differs with the primary particle size, the complete UV filter combination has to be rebalanced to achieve the same ratio of SPF and UVAPF.10
The SPF of a sunscreen formulation containing TiO2 UV absorbers is dependent on several factors, one being the ability of the TiO2 particles to absorb radiation in the UVB range. In this study, the observed efficiency of UV light absorption between 290 and 400 nm is elevated by the reduction of the primary particle size. The efficiency of particles to absorb electromagnetic radiation can be expressed as the absorption cross section. The bigger the absorption cross section, the more efficient light is absorbed by the particle. The absorption cross section is dependent on particle size, as described by Mie’s theory. The effect of particle size on the absorption cross section is welldocumented for pigments.11 This effect has also been demonstrated for UV radiation with sub-pigmentary TiO2.12
Conclusion
High temperature hydrolysis is a versatile method to synthesize TiO2 UV filters with different primary particle sizes. Subsequent application tests clearly show that increasing the primary particle size leads to unwanted effects such as strong whitening, lower SPFs and shifts in the absorption spectrum. These effects are already visible with primary particles below the regulatory threshold of 100 nm. This lowered performance with bigger primary particle sizes is not only critical in terms of customer acceptance, but brings along various formulation challenges. However, in this study, TiO2 UV filters with the smallest primary particle size displayed the best performance in application, combining low whitening with the highest in vitro SPF.
References
- Presentation of J. Augustin, B. Steinmetz “Klimawandel und UV-Strahlung, Wirkung auf die Entstehung von Hautkrebs in Deutschland,” available at www.bfr.bund.de/ cm/343/klimawandel_und_uv_strahlung.pdf (Accessed Mar 25, 2013)
- UV Filters in Sun Protection Products, opinion of the Federal Institute for Risk Assessment (BfR), Aug 6 2003, available at www.bfr. bund.de/cm/349/uv_filters_in_sun_production_ products.pdf (Accessed Mar 25, 2013)
- Sitzung der vorläufigen Kommission zur Bewertung von Kosmetischen Mitteln am 18. Nov 2004 und Apr 28 2005, Berlin, Federal Institute for Risk Assessment (BfR) 69 und 70, available at www.bfr.bund.de/ cm/343/69_und_70_sitzung_der_vorlaeufigen_ kommission_fuer_kosmetische_mittel. pdf (Accessed Mar 25, 2013)
- RC Weast, MJ Astle and WH Beyer, Handbook of Chemistry and Physics, 64th edn, CRC Press, Boca Raton (1983)
- K Mütze, Mie-Streung in ABC der Optik, Verlag Werner Dausien, Hanau (1961) S 533–535
- HG Völz, Pigments, Inorganic: General in Ullmann’s Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag, Weinheim Germany, available at dx.doi.org/10.1002/14356007. a20_243.pub3 (2009) (online)
- EU 1223/2009, Article 2.1 (k): “‘Nanomaterial’ means an insoluble or biopersistant and intentionally manufactured material with one or more external dimensions, or an internal structure, on the scale from 1 to 100 nm,” available at http://eur-lex.europa.eu/Lex- UriServ/LexUriServ.do?uri=OJ:L:2009:342:0 059:0209:EN:PDF (Accessed Mar 25, 2013)
- A McDougall, www.cosmeticsdesign-europe.com/On-your-radar/Nanotechnology/Studyfinds- industry-and-consumer-have-different- views-on-nanoparticles-in-sunscreen (Accessed Mar 25, 2013)
- Method for in vitro determination of UVA protection 2011, available at www. cosmeticseurope.eu/publications-cosmetics- europe-association/guidelines. html?view=item&id=33%3Amethod-for-invitro- determination-of-uva-protection- 2011&catid=46%3Aguidelines (Accessed Mar 21, 2013)
- 2006/647/EC, EU recommendation of Sep 22, 2006, on the efficacy of sunscreen products and the claims made relating thereto, available at http://eur-lex.europa.eu/LexUriServ/ LexUriServ.do?uri=OJ:L:2006:265:0039:004 3:en:PDF (Accessed Mar 21, 2013)
- A Brockes, Optik 21, ISSN 030-4026, (1964) pp 550–566
- M Zou et al, Nanotech 2010, vol 1, ISBN: 978-1-4398-3401-5, (2010) pp 510-513