Editor’s note: This is the final article in a three-part series discussing skin protectants. The first, published in October 2014, described practical and regulatory challenges. Part two, in November 2014, covered theoretical aspects and introduced insights in the area of perfluoropolyethers. This final part proposes a new view of the HLB system, bringing with it perfluoropolyethers as a novel solution to shield skin.
William C. Griffin must be given credit for developing criteria to create stable emulsions—i.e., the concept of hydrophilic/lipophilic balance (HLB). This system has successfully given formulators a well-defined paradigm with rules to rationalize their choice in emulsifiers, and more generally surfactants. Griffin explained the HLB system in his own words as follows. “Surfactants [including emulsifiers] consist of molecules that combine both hydrophilic and lipophilic groups (or polar and non-polar groups); it is the balance of the size and strength of these two opposing groups that we call HLB.”
Griffin’s proposed system became a paper at a 1949 symposium, then an article regarding the rules to select nonionic emulsifiers and surfactants produced by ethoxylation.1 In a second article (1954),2 Griffin added formulas to calculate the HLB of emulsifiers and oils.
Unfortunately, as many formulators know, these formulas do not work well for nonionic emulsifiers produced from propylene or butylene oxide, which are less hydrophilic than ethylene oxide. They do not work at all for ionic emulsifiers, since an ion contributes to hydrophilicity in an opposing way along a non-ionic chain; the smaller the size, the higher the contribution.
Some emulsifier manufacturers have tried to revive Griffin’s system. HLB values obtained by experimental methods (interfacial tension and dielectric constant measures, or chromatograph and nuclear magnetic resonance spectra) are reported in the literature—although, so are criticisms of the HLB system, particularly on the part of Pasquali.3-5
Griffin’s HLB system was developed for emulsifiers with the structure of amphiphiles, which typically have a hydrophilic (polar) head combined with a hydrophobic (non-polar) tail. It also is possible to have few polar heads and a single non-polar tail, or more complex polymeric structures, but it is difficult to apply the HLB concept.
In 1966, O’Lenick proposed a tridimensional HLB model for silicon-based emulsifiers to prepare three-component emulsions; i.e., water, hydrocarbon oils and silicon oils. This system was represented by a triangle where, at the vertices, each of these components theoretically would be present at 100%. Along the triangle sides, more realistic levels showed the balance of each component in relation to one another. The inside of the triangle therefore illustrated theoretical three-component emulsions.6
O’Lenick’s method has been considered by other authors, particularly by Somasundaran,7 but apparently it has not been accepted by major silicone manufacturers. For other polymeric emulsifiers, including perfluoropolyethers (PFPEs) and acrylics, the possibility to adapt Griffin’s HLB concept is precluded due to their structure, solubility and mechanism of action. Ironically, for the same reasons, these emulsifiers are central to the new paradigm proposed in this article.
Fluorophilicity: A New HLB System
As this series8, 9 has discussed, the problem of protecting skin has been unresolved for decades, with dermatologists apparently more dedicated to finding a solution than cosmetologists. Only partial success was achieved in the late 1980s when a limited range of Y-PFPEs, i.e., insoluble PFPEs produced from hexafluoropropene, was evaluated for skin protection as ingredients of emulsions prepared in accordance with the rules of the existing HLB paradigm.10-14
Thinking differently from the established HLB system, one can use the same HLB acronym, but with a different meaning, focus and rationale: the “hydrophilic/lipophilic” dichotomy of emulsifiers in Griffin’s system could instead be considered the “hydrophobic/lipophobic balance” of finished products. This new approach is no longer dichotomous since the two performances are not strictly related, which makes it possible to significantly increase water repellence together with oil repellence. However, there is one stipulation: fluorophilicity must be imparted to the finished product.
The concept of fluorophilicity/fluorophobicity depends upon the context. It is common in drug development, where the property of a fluorine-containing molecule is measured by its partition in the biphasic solvent system of perfluorodimethylcyclohexane and toluene. But it is uncommon in cosmetics, where it is instead a property achieved by the presence of a perfluorinated material in a composition.
Differently from drugs, in cosmetics there is no interest in measuring the fluorophilicity of an ingredient or finished product, rather its split between hydrophobicity and lipophobicity, with separate measures. To observe the concept in cosmetics, it is necessary to consider that in a composition with a certain fluorophilic character, lipophobicity is neither a synonym of hydrophilicity, nor does it oppose hydrophilicity, as commonly occurs. Analogously, hydrophobicity is not a synonym for lipophobicity, nor does it oppose hydrophilicity. Therefore, it is possible to maximize the former, while preserving the latter and vice versa.
Thus, the new and old paradigms are mutually exclusive. In fact, the two HLB approaches are not even related—while the old HLB of an emulsifier is calculated, the values of the new HLB of finished products result from experimental evaluations measuring, for example, the contact angle against water and/or oil, or other methods to measure hydrophobicity and lipophobicity.
Therefore, in the new paradigm, the term balance refers to the optimization of one performance, e.g., hydrophobicity (water repellence), without a corresponding sacrifice of the other performance, e.g., lipophobicity (oil repellence). As an aside, the choice between using the terms hydrophobicity/lipophobicity or water/oil repellence depends on the market sector, since the terms are equivalent. In this article, hydrophobicity/lipophobicity has been preferred for a formal analogy with the system of Griffin.
These desired performances clearly should be related to the desired level and type of skin protection and depend on the modified PFPE and the other ingredients. While this new paradigm requires further investigation to articulate, consolidate and identify its rules, the main promise is clear: to achieve effective skin protectants by exploiting a true shielding mechanism.
Usually, an emerging paradigm arises from a new and highly unexpected fact. Considering the insolubility of alkaline fluorides and of the small molecules of fluoro-organics—which is independent of the nature of fluorine bonds (ionic or covalent) and molecular weight—no chemist in their right mind would think the preparation of water or oil solutions with a high fluorine content (5-10%) by dissolving polymeric highly fluorinated materials to be possible. This is, indeed, a “revolutionary” fact.
Shielding Skin with New Emulsifiers
The barrier function of skin resides primarily in the top layer of the epidermis, the stratum corneum (SC). It is a barrier against both the diffusion of water from the skin, preventing dehydration and against aggressions from the external world. This barrier contains hydrophilic keratinocytes and lipophilic lipids, with other components influencing the water balance and natural moisturizing process.
Similarly, common emulsions are combinations of hydrophilic and lipophilic substances held together by emulsifiers having an amphiphilic structure, usually a hydrophilic head and a lipophilic tail. With this composition, common emulsions are compatible with the skin and effective in the restoration and preservation of the SC through moisturizing mechanisms. However, these compositions cannot defend against water-soluble or oil-soluble irritants; they may even act as a vehicle for some. To impart an effective shield, a new performance profile is required.
Chemically modified PFPEs are a new class of emulsifiers due to an uncommon structure that allows a new mechanism of emulsification combined with effectiveness in skin protection. The combination of hydrophobicity/lipophobicity of the chain with the hydrophilicity (or lipophilicity) of the chain ends is the key difference from the usual combination of hydrophilicity and lipophilicity of common emulsifiers (see Figure 1).
The solubility of these new emulsifiers suggests a novel mechanism of emulsion stabilization consisting in the enrichment of the fluorinated molecule near to the oil/water interface, but within the bulk of the phase where the modified PFPE is dissolved.
New Formula Design
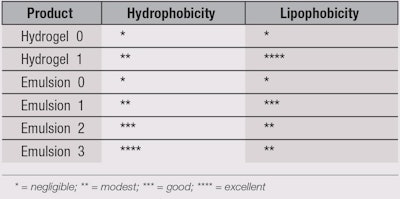
Based on the above discussion, formulating skin-shielding products is not as simple as adding hydrophobic/lipophobic materials to a base emulsion, as the old HLB paradigm suggests. For example, micronized PTFE powders (76% fluorine content) do not impart fluorophilicity and therefore, neither hydrophobicity nor lipophobicity, to a composition. Improved results are obtained by replacing insoluble PFPEs and common emulsifiers with soluble, chemically modified PFPEs15, 16 and carefully selecting the other ingredients (excluding common emulsifiers and a minimum content of hydrophilic viscosity agents). Thus, the design of the formulation on the whole and the structural agents determine the hydrophobicity/water repellence and lipophobicity/oil repellence of a product. Table 1 illustrates the characteristics of hydrogels and emulsions without and with a (chemically) modified PFPE. These observations were evaluated by contact angle measurements. Following are the tested compositions.
Hydrogel 0: 99.5% water, 0.5% acrylic viscosity agent and sodium hydroxide;
Hydrogel 1: 98.5% water, 1.0% PFPE phosphate, 0.5% acrylic viscosity agent and sodium hydroxide;
Emulsion 0 (o/w): 84.6% water, 15.0% emollients, 0.4% acrylic emulsifier and sodium hydroxide;
Emulsion 1 (o/w): 84.6% water, 14.0% emollients, 1.0% PFPE phosphate, 0.4% acrylic emulsifier and sodium hydroxide;
Emulsion 2 (o/w): 84.6% water, 14.0% emollients, 1.0% PFPE stearylamide, 0.4% acrylic emulsifier and sodium hydroxide; and
Emulsion 3 (w/o/w): 84.6% water, 14.0% emollients, 1.0% PFPE stearylamide, 0.4% acrylic emulsifier and sodium hydroxide.
It should be noted that emulsions are considered the most suitable technical form for skin protection, only for reasons of continuity. Hydro-gels are also a convenient technical form, particularly to work against oil-soluble irritants.
New HLB Methods
As mentioned, the new HLB paradigm requires the development of evaluation methods, but currently, such methods do not exist for the cosmetics industry. On the contrary, perfluorosurfactants and perfluoropolymers have been used for years in the textile industry, so this may be a place to start.
In textiles, liquid repellence means benefits for the wearer such as protection from rain and snow, faster drying times and longer-lasting cleanliness while keeping breathability. Perfluorosurfactants and perfluoropolymers have therefore been assessed for the durability of their water/oil repellence and minimal impact on other performances.
Test methods for textiles were proposed by the American Association of Textile Chemist and Colorists (AATCC) in the area of resistance to wetting and penetration of water and oil. In 2012, work by an AATCC committee was completed and a test method was published.17 To evaluate resistance to wetting by aqueous solutions, drops of standard test liquids, consisting of a selected series of water/alcohol solutions with varying surface tension, are placed on the fabric surface and observed for wetting. An arbitrary rating of 10, at 20% water and 80% alcohol, is considered the highest or most repellent; the minimum acceptable rating is typically 4 or 5, after multiple launderings.
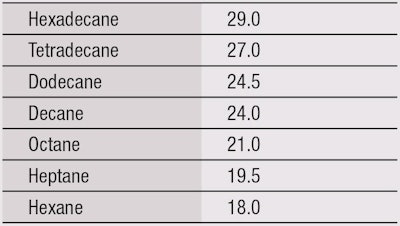
Similarly, an oil repellence test method measures the resistance to wetting by a series of hydrocarbons with varying surface tensions.18 Again, the arbitrary oil repellence rating is the number of the liquid with the lowest surface tension, which does not wet the substrate. The maximum rating is 8 (see Table 2).
However, these test methods are highly specific and related to certain substrates in order to maximize a precise performance. Therefore, the transfer to cosmetics cannot be immediate.
Some work to develop methods specifically for cosmetics has been performed by Ausimont/Solvay Solexis, the results of which have been published.19 Specifically, a drop of liquid, i.e., water or a vegetable oil, is applied on a paper filter pre-treated with a cosmetic emulsion or hydrogel and the time it takes for complete penetration is measured. The water/oil repellence of hydrogels and emulsions also were evaluated by static contact angle measures using a non-polar liquid, n-hexadecane and water.20, 21
It is practically impossible to evaluate skin protectants for their whole potential and independently of their mechanisms of action—especially with the objective to compare developmental and commercial products. While the ability of a product to improve skin moisture content can be evaluated with established dermatological methods, for actual protective performance based on a shielding mechanism, it is advantageous to refer to more objective physico-chemical properties of finished products. Previous articles in this series8, 9 showed the need for this segmentation.
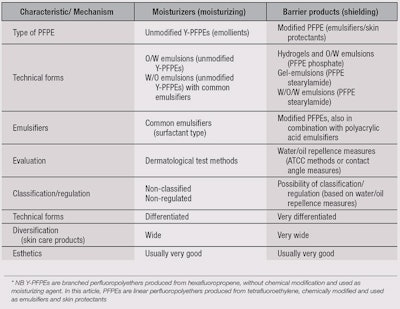
Table 3 illustrates a comparison between the existing paradigm—where an unmodified PFPE is added to a base emulsion prepared with common emulsifiers, which is focused on dermatological evaluations, and the emerging paradigm—where modified PFPEs work as emulsifying and protective agents, which is focused on physico-chemical measures.
Indeed, it seems more convenient to develop specific rules for a new HLB system focused on the relatively easy evaluation of these properties, rather than the difficult evaluation of skin conditions (see Figure 2).
In the existing paradigm, one typically prepares products without dermatologic feedback. Then, evaluations of their performance on skin are made. Such evaluations depend on irritants as well as individual volunteers’ reactions. In addition, product adjustments must then be re-tested on subjects.
With the new paradigm, products could be prepared and their performances measured and adjusted without the involvement of dermatologic feedback—until good in vitro performances are achieved. Of course, investigations are still necessary to standardize the process, in order to ensure good correlation between physico-chemical measurements and protection factors. Note, investigations by dermatologists based on the existing paradigm also do not provide an objective “skin protection factor” rating, whereas under the new paradigm, ratings for protection against oil-soluble or water-soluble irritants are possible. Thus, with this new iterative process, it is possible to directly correlate skin protectants with evaluation methods, which is missing from Griffin’s system.
Conclusions
In the newly proposed paradigm, HLB stands for the hydrophobic/lipophobic balance—not of an individual emulsifier, but of a product. Measures of hydrophobicity and lipophobicity are assumed to indicate the shielding potential of a finished product against water- and oil-soluble irritants. This mechanism of protection allows for a new classification that could be the base for a new regulation (refer to Part I in this series for this discussion).
Sunscreens offer a regulatory model from which to work with, although skin protectants are comparatively either more complex for several reasons or simpler due to the option of referencing in vitro tests. Analogously to sunscreens, which protect against both UVA and UVB, skin protectants should protect against oil- and water-soluble irritants. Of course, this distinction is artificial and probably not completely adequate.
Nevertheless, this approach has the potential to produce the data necessary for product developers to compare development products, as well as dermatologists and consumers to choose a suitable protectant.
It is important to stress, once again, this emerging paradigm is unrefined and will require progressive research to define the rules in detail. Besides, its articulation could be a guide for researching in other areas of cosmetics and revising investigations of the past.
References
- WC Griffin, J Soc Cosmet Chem 1 311-26 (1949)
- WC Griffin, J Soc Cosmet Chem 5 249-56 (1954)
- R Pasquali, N Sacco and C Bregni, Lat Am J Pharm 28(2) 313-7 (2009)
- R Pasquali, M Taurozzi and C Bregni, Int J Pharm 356 44-51 (2008)
- R Pasquali, N Sacco and C Bregni, J Disp Sci Tech 31(4) 479-481 (2010)
- AJ O’Lenick Jr and JK Parkinson, Cosm & Toil 111(10) 37-44 (1996)
- P Somasundaran, C Somil and P Puroit, Adv Colloid Interface Sci 128 103-109 (2006)
- www.cosmeticsandtoiletries.com/research/chemistry/Problems-with-Skin-Protectants-Part-I-A-Discussion-premium-275474601.html
- www.cosmeticsandtoiletries.com/formulating/function/filmformer/Problems-with-Skin-Protectants-Part-2-Theory-and-Perfluoropolyether-Insightspremium-283343241.html
- R Forestieri, F Brunetta and G Pantini, 2nd International Meeting on Cosmetic Dermatology, Rome (May 20-22, 1987)
- G Pantini and PL Bencini, Drug Cosmet Ind 2 29-32 (1989)
- PL Bencini, C Crosti, F Brunetta and G Pantini, Int J cosmet Sci 12 273-79 (1990)
- PL Bencini et al, Drug Cosmet Ind 2 28-32 (1990)
- C Crosti, PL Bencini, F Brunetta and G Pantini, SÖFW J 16 1020-23 (1992)
- G Pantini, R Ingoglia and F Brunetta, Cosm & Toil 116(8) 81-92 (2001)
- G Pantini and R Ingoglia, IFSCC Conference, Seoul (2003)
- Aqueous Repellence: Water/Alcohol Solution Resistant Test (193), AATCC Technical Manual, Weston Parkway, NC (2012)
- Oil Repellence: Hydrocarbon Resistant Test (118), AATCC Technical Manual, Weston Parkway, NC (1997)
- G Pantini et al, IFSCC Conference, Osaka (2006)
- www.krauss.de
- Y Yuan and TR Lee, Surface Science Techniques, Springer, Heidelberg, Germany (2013)