Skin aging is characterized by progressive changes in the regulation of cellular processes and miscommunication between cells and their immediate environment, the extracellular matrix (ECM). These malfunctions, which may result from physiological and/or environmental events, are most notably characterized by the increased activities of metalloproteinases (MMPs) and the reduced synthesis of their endogenous inhibitors, i.e., tissue MMP inhibitors (TIMPs),1–5 as well as the reduced biosynthesis of major ECM proteins.6 As a result, the extracellular matrix is no longer renewed and the skin shows the effects of aging.
In relation, levels of the cellular components involved in interactions between cells and their matrices are altered with skin aging and are responsible for less efficient interactions between cutaneous cells and the ECM. In addition, the regulation of many glyco-proteins changes with age, which impacts cellular functions. For example, syndecans such as transmembrane heparin sulfate proteoglycans participate in focal adhesion formation7 and play an important role in skin homeostasis by acting as co-receptors for growth factors.8 However, with age, syndecan expression decreases in keratinocytes9 and skin.10
Aside from these modifications of ECM macromolecules and molecules implicated in cell matrix interactions, age-related changes in cells themselves also must be considered. Recent work has shown that increased progerin synthesis, in conjunction with the shortening of telomeres, leads to cellular senescence in normal human fibroblasts.11 Thus, targeting such cellular impairment provides an interesting development path for anti-aging compounds. Many key roles of endogenous tripeptides such as glutathione, thyrotropin-releasing hormones and other tripeptides12 have been identified, and since the body uses tripeptides for cell communication, these compounds have been proposed as anti-aging agents to boost healthy skin functions and reverse skin damage mostly by enhancing fibroblastic collagen production.13 However, while most of the published data shows that these compounds may increase collagen synthesis, little is known about other properties such as their role in cellular aging.
The present study therefore looks at the broader anti-aging properties of tripeptides by specifically examining the effects of trifluoroacetyl-tripeptide-2a (TT2) on ECM protection, on the synthesis of proteoglycans in cell-matrix interactions, and on the synthesis of progerin, a protein recently identified as a co-inducer of cellular senescence. This ingredient was chosen as the best candidate based on the in vitro screening of a library of materials built from peptides having known physiological activity. In vitro studies were then conducted to assess the ability of this tripeptide to inhibit MMP-1, MMP-3, MMP-9 and elastase, to stimulate syndecan-1 expression in human keratinocytes, and to reduce progerin synthesis in mature human normal fibroblasts. Two in vivo studies were conducted to confirm in vitro findings using cutometry and fringe projection profilometry techniques.
Materials and Methods
MMP-1, MMP-3 and MMP-9 assays: Extracts of MMP-1, MMP-3 and MMP-9 were calibrated for their specific inhibition capacity and incubated in the absence (control) or presence of the fluorogenic substrate, and in the absence or presence of the TT2 at different concentrations for 30 min. For MMP-1 inhibition, the TT2 was tested at 0.08 ppm and 0.25 ppm; for MMP-3 inhibition, 0.001 ppm, 0.003 ppm, 0.01 ppm and 0.03 ppm; and for MMP-1 inhibition, 0.08 ppm, 0.25 ppm and 0.75 ppm. Concentrations were chose depending on the initial peptide concentration, which was diluted in serial dilution. At the end of the incubation period, the fluorescence of the assay media was measured using a microplate reader and the following equation was applied:
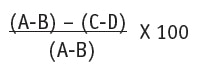
Eq. 1
where A = substrate + MMP; B = substrate alone, C = substrate + MMP + sample; and D = substrate + sample. This equation gives the percent activity of each MMP. From this equation, the percent inhibition is then calculated, i.e., percent inhibition = 100 - percent activation. This indicates the activity of the peptide in vitro and provides a way to calculate the activity of the peptide on MMP inhibition.
Collagenase and elastase assays: Slices of human skin 5 x 2 mm and 5 μm thick were pre-incubated 30 min in the absence or presence of the TT2 at 0.05 ppm. Slices were then incubated for 3 hr in the absence (control) or presence of type I collagenase or leucocyte-derived human elastase. At the end of the incubation period, collagen or elastic fibers were stained with Masson’s trichrome or orceine, respectively, as previously described.14 The inhibitory effects of the tripeptide on collagenase and elastase activities under various conditions were evaluated by quantifying the surface occupied by collagen or elastic fibers in 40 randomly selected microscope fields.
Syndecan-1 expression: Human keratinocytes were incubated for 24 hr with TT2 at various concentrations; the most significant results were found with concentrations of 0.0005 ppm and 0.005 ppm. After the incubation period, cells were fixed and incubated with the primary anti-syndecan-1 antibody, followed by the FITC-coupled secondary antibody control. The fluorescent signal was then analyzed using an optical microscope and software.
Progerin ELISA assay: Human normal fibroblasts from a 44-year-old Caucasian donor were incubated for 96 hr with 0.005 ppm and 0.05 ppm of the TT2. At the end of the incubation period, progerin was quantified following the method published by Verdy.15 Total protein content of the cells was assessed in cell lysates obtained by sonication and using the Bradford method.16
In vivo jawline measurements: Ten healthy volunteers, ages 54–64 (mean = 59), were included in an in vivo jawline study. In a split-face test, for 56 days, the volunteers applied typical usage amounts of both a test and placebo emulsion cream—i.e., containing either 4 ppm of the TT2 or a placebo—on separate halves of the face and along the jawline and massaged the areas until the cream completely absorbed into the skin (see Formula 1). The creams were applied twice daily, i.e., morning and evening, and subjects washed their hands between applications. At days 0, 28 and 56, the jaw line volume of every volunteer was measured for sagging using a fringe projection profilometric technique.17
In vivo cutaneous firmness and elasticity—cutometry: Thirteen healthy volunteers ranging in age from 54–66 (mean age = 60) were included in an in vivo firmness and elasticity study. For 28 days, each volunteer used the same test emulsion cream (see Formula 1) containing either 4 ppm of the TT2 or a placebo. The product application protocol was randomized and samples were again applied to half of the face and neck daily, i.e., morning and evening. At days 0 and 28, the cutaneous firmness and elasticity of every volunteer’s cheeks were measured by cutometerb. The cutometer briefly created negative pressure on the skin surface (300 mbar) and measurements were taken of the vertical movement on the induced skin to determine three main parameters: firmness (= extensibility, tightness, tonicity), elasticity and visco-elasticity (= plasticity).
Statistics: With all tests, the data is expressed as the mean ± the standard error (S.E.) of results obtained at least in triplicate, as indicated. The statistical significance of the data was calculated using the student’s t-test, paired student’s t-tests, or by one-way analysis of variance (one way ANOVA) followed by Holm-Sidak’s tests or All Pairwise Multiple Comparison Procedures (Fisher LSD Method), as indicated.
Results and Discussion
The first part of this study measuring the effect of the tripeptide on MMP-1, MMP-3 and MMP-9 activities indicated that TT2 can protect against age-related extracellular matrix degradation. Figure 1, Figure 2 and Figure 3 show the tripeptide significantly inhibited MMP-1, MMP-3 and MMP-9 activities, in a dose-dependent manner, in comparison with the vehicle alone, which had no effect (data not shown). In human skin slices incubated with collagenase or elastase, the tripeptide was shown to efficiently protect the collagen and elastic fibers in skin from degradation by about 43% and 100%, respectively (see Figure 4 and Figure 5). This was in comparison with two reference inhibitors as the control: elastinal for the elastase assay, and 1.10 phenantroline for the collagenases assay. Both active references confirmed the experimental model (data not shown).
In addition, since age-related human skin deterioration also involves less efficient communication between skin cells and the extracellular matrix, the second part of the study examined the effects of TT2 on proteoglycans. Proteoglycans are important to cell-matrix adhesion. In cell biology, focal adhesions or cell-matrix adhesions are specific types of large macromolecular assemblies through which both mechanical force and regulatory signals are transmitted. Wegrowski et al., in 2005, and Oh et al., in 2011, demonstrated that with increasing age, syndecan-1 synthesis decreases in keratinocytes18 and in skin.19 Syndecan-1 is also credited with maintaining skin homeostasis, in particular as a co-receptor for various growth factors.20
As shown in Figure 6, TT2 significantly increased the syndecan-1 synthesis in human keratinocyte monolayers. This stimulatory effect of the tripeptide at concentrations of 0.0005 ppm and 0.005 ppm was approximately +19% and +56% (p < 0.05), respectively, and was equivalent to the effects obtained with TGF-β, a well-known inducer of syndecan synthesis.21 This suggests that TT2 could potentially reverse the effects of aging. Taken together, these results prove that the tested tripeptide exhibits activity to protect the extracellular matrix, maintain the healthy interactions between cells and their surrounding matrices, and stimulate the metabolic pathways of skin cells. Based on these conclusions, the authors sought to study the activity of TT2 on mechanisms that, according to recent theory, are involved in triggering cellular senescence; specifically evaluated were the effects of the tripeptide on progerin synthesis in fibroblasts.
Progerin Synthesis in Fibroblasts
In 2006, Mistelli et al. showed that progerin, a truncated form of nuclear lamin A, is more abundant in the skin of older subjects by comparison with younger subjects.22 In 2011, Verdy et al. quantitatively determined the progerin level in in vitro cultures of fibroblasts from young and old donors. These results reinforced the idea that progerin levels increase with age,23 and in 2011, Cao et al. showed that progerin and the effects of shorter telomeres together trigger cellular senescence in normal human fibroblasts.24 Directed by the results of these studies, the authors evaluated the potential effects of TT2 on progerin synthesis in old fibroblast cell cultures via progerin ELISA assay, as previously described.
As shown in Figure 7, at concentrations of 0.005 ppm or 0.05 ppm, TT2 significantly reduced progerin synthesis in fibroblasts by 18.0% (p < 0.05) and 21.9% (p < 0.05), respectively. While TT2 may conceivably act in several ways to reduce progerin synthesis in fibroblasts, since results were observed in as little as 48 hr, it is likely the tripeptide influences the farnesyl transferase inhibitor,25 which is responsible for the processing of lamin A to progerin. Progerin undergoes farnesylation at a carboxyterminal CaaX motif, but it lacks the cleavage site for the endoprotease ZMPSTE24 and therefore cannot be further processed to mature lamin A. Here, the peptide was able to decrease the progerin production in mature fibroblasts, so it is proposed the Lamin A processing was not further disturbed. Additional studies are under way to substantiate this hypothesis.
In vivo Analyses
Aside from these in vitro studies, the effects of TT2 were evaluated in two in vivo studies—one examining its effects on sagging along the jawline of volunteers via fringe projection profilometry, and the other examining the mechanical properties of firmness, elasticity and visco-elasticity via cutometry. As shown in Figure 8, TT2 significantly reduced the volume of the jawlines in healthy volunteers after 4 weeks and 8 weeks of treatment by -0.6% and –3.4%, respectively (p < 0.05 vs T0 value and vs placebo); the placebo-treated half of the face showed no effect or even worse, sagging.
As shown in Figure 9, the tripeptide improved cutaneous firmness (+20.0%, p < 0.1), elasticity (+20.9%, p < 0.1) and visco-elasticity (+13.3%, p < 0.01). On the side treated with the placebo cream, a slight but insignificant increase in cutaneous firmness (+2.7%) and elasticity (+3.5%) were observed, as well as a slight but insignificant decrease in cutaneous visco-elasticity (-1.4%). Further, as shown in Figure 10, the anti-wrinkle and anti-sagging effects of the treatment with TT2 were confirmed by facial macrophotography after 28 days and 56 days of treatment, whereas the placebo-treated side showed no effect.
Formulation Guidelines
Since TT2 is composed of hydrophilic natural amino acids, it is fully soluble in aqueous medium, glycerin, glycol and ethanol. Also, as established by laboratory tests, TT2 is stable at pH values ranging from 3.0 to 6.5 (data not shown) and is compatible with most types of emulsifiers and thickeners. In order to maximize its stability in formulas, addition at the end of the process (35–40°C) is recommended.
Conclusion
In conclusion, the effects of the synthetic tripeptide TT2 were confirmed to regulate biological mechanisms involved in the aging process—i.e., the inhibition of proteases implicated in degradation of the ECM, and the increase in the expression of syndecan-1. Further, and most interestingly, the research presented here is the first exploring the effects of a tripeptide on progerin production, which is implicated in cellular senescence. TT2 also showed positive and significant effects on signs of aging in two in vivo studies, including reducing the appearance of skin sagging and slacking, and improving firmness, elasticity and visco-elasticity in volunteers treated with the peptide formulation, in comparison with a placebo formulation. These results demonstrate that TT2 has significant and progressive effects on the most important signs of aging, i.e., wrinkles, firmness, elasticity and sagging, after only 28 days treatment. In addition, a complete evaluation of the safety of the TT2 showed the peptide to exhibit an excellent safety profile (data not shown).
References
- W Hornebeck, Down-regulation of tissue inhibitor of matrix metalloprotease-1 (TIMP-1) in aged human skin contributes to matrix degradation and impaired cell growth and survival, Pathol Biol 51, 569–573 (2003)
- L Rittie and G Fisher, UV-light-induced signal cascades and skin aging, Ageing Res Rev 1, 705–720 (2002)
- B Sela, Dermatological manifestations of smoking, Harefuah 141, 736–740 (2002)
- K Nelson and J Melendez, Mitochondrial redox control of matrix metalloproteinases, Free Radic Biol Med 37, 768–784 (2004)
- S Pillai, C Oresajo and J Hayward, Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review, Int J Cosmet Sci 27, 17–34 (2005)
- L Robert, J Labat-Robert and A Robert, Physiology of skin aging, Pathol Biol 57, 336–341 (2009)
- L Carvallo et al, Non-canonical Wnt signaling induces ubiquitination and degradation of syndecan-4, J Biol Chem 285, 29546–29555 (2010)
- M Bass and M Humphries, Cytoplasmic interactions of syndecan-4 orchestrate adhesion receptor and growth factor receptor signaling, Biochem J 368, 1–15 (2002)
- Y Wegrowski, L Danoux, J Contet-Audonneau, G Pauly and F Maquart, Decreased syndecan-1 expression by human keratinocytes during skin aging, J Invest Dermatol 125 A5 (2005)
- J Oh, Y Kim, J Jung, J Shin and J Chung, Changes in glycosaminoglycans and related proteoglycans in intrinsically aged human skin in vivo, Exp Dermatol 20, 454–456 (2011)
- K Cao et al, Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts, J Clin Invest 121, 2833–2844 (2011)
- J Geleijnse and M Engberink, Lactopeptides and human blood pressure, Curr Opin Lipidol 21, 58–63 (2010)
- F Maquart et al, In vivo stimulation of connective tissue accumulation by the tripeptide-copper complex glycyl-L-histidyl-Llysine- Cu2+ in rat experimental wounds, J Clin Invest 92, 2368–2376 (1993)
- JD Bancroft and A Stevens, Theory and Practice of Histological Techniques, 4th edn, Churchill and Livingstone, eds (1996) pp 129
- C Verdy, J-E Branka and N Mekideche, Quantitative assessment of lactate and progerin production in normal human cutaneous cells during normal ageing: Effect of an Alaria esculenta extract, Int J Cosmet Sci 3, 1–5 (2011)
- M Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal Biochem 72, 248–254 (1976)
- J Lagarde, C Rouvrais, D Black, S Diridollou and Y Gall, Skin topography measurement by interference fringe projection: A technical validation, Skin Res Technol 7, 112–121 (2001)
- Y Wegrowski, L Danoux, J Contet-Audonneau, G Pauly and F Maquart, Decreased syndecan-1 expression by human keratinocytes during skin aging, J Invest Dermatol 125, A5 (2005)
- J Oh, Y Kim, J Jung, J Shin and J Chung, Changes in glycosaminoglycans and related proteoglycans in intrinsically aged human skin in vivo, Exp Dermatol 20, 454–456 (2011)
- M Bass and M Humphries, Cytoplasmic interactions of syndecan-4 orchestrate adhesion receptor and growth factor receptor signaling, Biochem J 368, 1–15 (2002)
- JF Manakil, GJ Seymour and PM Bartold, Effect of cytokine and antigen stimulation on peripheral blood lymphocyte syndecan-1 expression, Oral Microbiol Immunol 22(4) 272–276 (2007)
- P Scaffidi and T Misteli, Lamin A-dependent nuclear defects in human aging, Science 19 312(5776) 1059–1063 (2006)
- C Verdy, J-E Branka and N Mekideche, Quantitative assessment of lactate and progerin production in normal human cutaneous cells during normal ageing: Effect of an Alaria esculenta extract, Int J Cosmet Sci 3, 1–5 (2011)
- K Cao et al, Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts, J Clin Invest 121, 2833–2844 (2011)
- SH Yang et al, A farnesyl transferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation, J Clin Invest 116, 2115–2121 (2006)










