Dry skin is a common complaint by men and women alike and its incidence and severity increase with age. This condition is the result of an impaired barrier function, increased transepidermal water loss (TEWL) and a significantly lower level of ceramides in the horny layer that causes the skin to lose an excessive amount of water. This inability to retain moisture causes the skin to look dull and flaky, feel rough and lose elasticity. Dry skin can be due to genetic and metabolic factors, poor diet and certain conditions such as dermatitis, eczema or seborrhoea; however, in many cases, this type of skin is the result of chronic irritation by environmental and lifestyle factors.1 The conventional treatment of dry skin is to apply moisturizer or skin cream. These products work primarily by blocking the surface of the skin to increase the water content in the stratum corneum (SC), thus producing a state of hydration and imparting a temporary barrier to damaged SC.
Gamma-linolenic acid (GLA) has been suspected to be a conditionally essential component for optimal structure and function of the skin. That is, under certain conditions its requirement may exceed the individual’s capacity to synthesize this fatty acid and therefore has to be supplied pre-formed. GLA has been found in some vegetable oils such as evening primrose (EPO) and borage oil. These oils have been reported to have a skin barrier repairing effect, to normalize excessive transepidermal water loss, and to improve skin smoothness parameters after topical administration to healthy and irritated skin.2
A recently published open-labelled study with elderly people using borage oil and a randomized, double-blind, placebo-controlled study with healthy adults using EPO4 not only confirmed these findings after systemic application, but also showed that oral supplementation could improve various biophysical skin parameters that are indicators of age-related structural and functional changes in healthy skin tissues. In view of the growing interest of the cosmetics industry in nutricosmetics and the widespread occurrence of stressed and dry skin, the present study was designed to test whether systemic EPO could be reproduced in a human model of irritated skin.
Materials and Methods
The randomized, placebo-controlled, double-blind, parallel group trial was performed by a third partya.
The methodology applied was identical to that described in detail by Muggli.4 A total of 40 healthy females and males were recruited. Criteria for eligibility were: ≥ 18 years old, clinically healthy, and with self-assessed dry and rough skin. Exclusion criteria were pregnancy and any type of skin disease.
The subjects were randomly allocated to either EPO or placebo treatment. The subjects were between 32–60 years old; there was no significant difference between the age means of the two treatment groups (45.3 vs. 46.4 years). The subjects were instructed not to use topical preparations on the test areas (inner sides of both forearms) beginning seven days before the study and lasting through the end of the study. For cleansing, only water or a mild syndetb were allowed.
Experimental skin irritation was induced with sodium dodecyl sulphate (SDS). After the baseline measurement, a cotton pad soaked with a 2% solution of SDS in distilled water was applied to the test areas on the inner sides of both forearms. After 24 hr, the test patch was removed, any remaining SDS solution was dabbed with a soft paper towel, and the skin was rinsed with tap water and gently blotted dry with a soft paper towel.
Measurements were taken before treatment (day 0), 4 hr after removal of the SDS test patch (day 1), and after EPO treatment at days 28 and 84. Before each measurement the subjects were adapted for 30 min to a room temperature of 21±1°C and a relative humidity of 50±5%.
Oral treatment: The oral treatment comprised six 500-mg capsules, totalling for the active group 3g EPO, and providing 345 mg of GLA per day. The subjects were instructed to take three capsules twice daily; in the morning and evening during meals for 84 days.
The identically looking 500-mg soft gelatine capsules were manufactured for the evening primrose and the placebo group. The evening primrose oilc and placebo oild were stabilized with 8 mg of dl-α-tocopherol acetate.
The GLA label claim per capsule was 57.5 mg; SDSe was obtained for the study.
Measurements
The following skin parameters were measured: moisture, redness, TEWL, elasticity, firmness, fatigue resistance and roughness.
Skin moisture: The skin moisture content was measured with a Corneometerf to determine the electrical capacitance of the skin surface in arbitrary units.5 Three separate measurements on adjacent skin sites were performed, and the mean was used to define the hydration state of the SC.
TEWL: The TEWL was measured with a Tewameterg. The Tewameter measures the water evaporation through the skin based on Fick’s diffusion principle in g m-2 hr-1. The measurements were performed after the guidelines of the Standardization Group of the European Society of Contact Dermatitis.6 Each value was the average of three different measurements on adjacent skin sites.
Skin redness: Skin color was measured with a chromameterh in compliance with the recommendations of the International Commission on Illumination (CIE) and according to the guidelines of the Standardization Group of the European Society of Contact Dermatitis.7
Three separate measurements were performed on adjacent skin sites and the mean was used to define the degree of skin redness.
Biomechanical skin properties: The biomechanical properties of the skin were assessed with a Cutometerj. This measurement is based on the suction principle.8 By applying a defined underpressure, skin is drawn into a hollow tube with an orifice of 2 mm in diameter. The skin then is allowed to retract at ambient pressure. The penetration depth of the skin into the tube is continuously recorded optically and frictionless as a function of time. From the penetration depth curve, the three biomechanical skin parameters can be calculated: 1) skin firmness, 2) skin elasticity, and 3) skin fatigue resistance. The study was conducted with 20 successive measurement cycles of 1-sec suction (450 mbar) and 1-sec retraction. The calculations were performed with softwarek.
Skin roughness: Skin roughness was measured with phase-shifting rapid in vivo measurement of skin (PRIMOS), a noncontact measurement device that allows for real-time three-dimensional in vivo measurement of the human skin microtopography based on active image triangulation.9 Skin roughness was assessed by means of the parameter RZ (mean depth of roughness) defined as:
where n is the number of equal segments into which the scan length, l, has been divided into, and RZi is the maximum peak to valley depth within each individual segment.
Biometry: All measurements were automatically recorded, then checked for validity and quality and stored centrally in a database. The data was evaluated with softwarem.
The data was not necessarily derived from a population of normally distributed values and it was a relatively small sample size; thus, the two treatment groups were compared with the Wilcoxon signed-rank test on differences. No correction for multiple variables was applied. Differences were considered significant at P-values <0.05 (two-sided).
Results
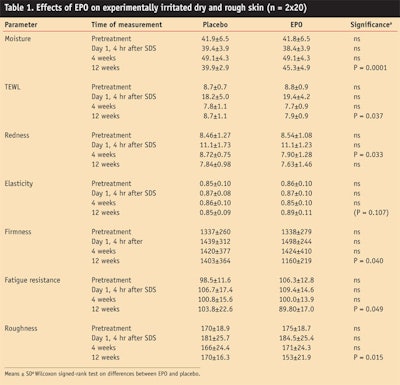
The oral supplements were well-tolerated. Seven volunteers from the EPO and two volunteers from the placebo group reported transient gastrointestinal discomfort at the beginning of the study. None of the volunteers withdrew from the study. The complete set of planned measurements could be obtained from all volunteers. The data is presented as group means in units as measured (see Table 1) and as percent change of the pretreatment value (see Figure 1).
The mean values of the two treatment groups at baseline did not differ significantly from each other and were very similar. This indicates that the EPO and the placebo group were highly comparable with respect to their skin properties. Artificial irritation of the skin with SDS led to expected higher TEWL and redness values. With the exception of moisture and TEWL at week 4, none of the mean values of the placebo group at weeks 4 and 12 differed significantly from the pre-treatment value. With the exception of moisture and TEWL, none of the mean values of the EPO treatment group at week 4 differed significantly from the pretreatment value. The values after EPO treatment at week 4 did not differ significantly from the corresponding values of the placebo group, with the exception of significantly higher redness value. The picture was completely different eight weeks later.
At week 12, the values of all skin parameters were better in the EPO group compared with the placebo group, with the exception of skin redness. Significant improvements were measured in terms of percent differences to the placebo: moisture, 13.9%; TEWL, 10.2%; firmness, 19.2%; fatigue resistance 22.2%; and roughness, 13.0%. The levels of statistical significances ranged between P=0.0001 and 0.049. The improvement of elasticity by 3.5% was borderline significant (0.107).
Discussion and Conclusions
One of the main functions of the skin is to protect against dehydration and penetration of external factors. This barrier function resides in the SC and is formed from protein-enriched corneocytes embedded in “cement” composed of lipids that are organized in extensive lamellar sheets. The lipids are important for the water barrier, whereas the corneocytes protect against exogenous compounds or physical injury. Barrier permeability depends on the composition of the SC lipids. Among the integral components of water barrier is the omega-6 fatty acid linoleic acid (LA). LA applied systemically or topically has been shown to regenerate a defective skin barrier in animals and humans deficient in essential fatty acids.10
The mechanism by which LA maintains skin integrity and function is not completely understood. LA is the prominent fatty acid in ceramides, the largest polar lipid class of the SC. Linoleic acid deficiency leads to disruption of the stacked lipid bilayers surrounding the corneocytes of the horny layer resulting in a breakdown of the barrier function.11 Apart from this structural function, LA has a metabolic role as the parent fatty acid of the long-chain polyunsaturated fatty acid GLA (C18:3n-6). GLA acid itself is rapidly and efficiently transformed to dihomo-gamma-linolenic acid (DGLA), the precursor of prostaglandin E1 and 15-hydroxy-eicosatrienoic acid, metabolites with potent anti-inflammatory effects.12
Furthermore, GLA suppresses, at least in vitro, the formation or release of IL-1β and TNF-α, both important inflammatory mediators.13–15 Consequently, a deficiency in LA and by implication that of GLA may not only disrupt the skin barrier but may predispose the skin to inflammatory overreactions; i.e., the skin reacts readily to minor insults with an overshooting or chronic inflammatory response.
Although LA deficiency is virtually nonexistent in industrialized societies, the skin still may be undersupplied with GLA. GLA is metabolized from its precursor LA by Δ-6-desaturase, a very susceptible enzyme whose activity can be impaired genetically by age as well as by several nutritional deficiencies and lifestyle habits.16–18 Thus, insufficient amounts of GLA may be produced by the body even in the presence of adequate LA. The skin is particularly sensitive to suboptimal GLA supply as it lacks the necessary Δ-6-desaturase enzyme with which to form it in situ. This could have profound effects on skin barrier function and on dermal immuno-inflammation. In fact, there is some evidence that the inflammatory component of neurodermitis, psoriasis and other skin affections might be the result of inadequate amounts of prostaglandin E1 and 15-hydroxy-eicosatrienoic due to the lack of the substrate GLA.12 Obviously the skin is an organ that seems to depend on the supply of preformed GLA.
Barrier permeability, and by consequence, skin moisture, is regulated physiologically but often is disturbed by man-made or natural stressors.1 UV radiation from the sun; contact with household chemicals; cosmetics; wind; air conditioning; smoking; and frequent bathing, showering or swimming, especially in strongly chlorinated hot or cold water, may all weaken the skin’s barrier function and increase TEWL. The result may be dry skin that provokes cutaneous discomfort and an unaesthetic appearance.
Experimentally, mechanical (tape stripping), chemical (acetone, SDS, detergents) or UV-induced stress has been shown to disrupt the cutaneous permeability barrier, to increase TEWL, to cause hyperproliferation of the skin and to trigger the production of inflammatory epidermal cytokines such as TNF and IL-1.19–21
All these phenomena are intensified with age and follow often a seasonal pattern. In winter, cold increases TEWL22 and can strip the skin of moisture which, combined with low humidity and indoor heating, can cause dryness, and even cracking and chapping. Low humidity amplifies the hyperproliferative and inflammatory response to barrier disruption23,24 and may explain the seasonal exacerbation of dry skin and cutaneous disorders such as atopic dermatitis and psoriasis, diseases that are characterized by a defective lipid barrier, epidermal hyperplasia and inflammation. If irritation of the skin, alone or on top of a restricted metabolism of LA to GLA, breaks down the skin’s lipid barrier and is the cause of dry skin, cutaneous hyperproliferation and inflammation, then GLA should produce relief.
Indeed, several animal and clinical studies have shown this to be the case. Oral administration of GLA to guinea pigs and rats leads to higher levels of this fatty acid in the structural lipids of the epidermis and to an increase of the anti-inflammatory mediators prostaglandin E1 and 15-hydroxy-eicosatrienoic acid in the epidermis.25–29 In animal models, injury to the skin barrier, manifested by an increased TEWL and other skin changes as the result of a fatty acid deficient diet or from application of a lipid disrupting detergent, could be reversed by oral treatment with GLA-rich vegetable oils.13, 25, 27, 30–31 Borage and evening primrose oil reversed experimental epidermal hyperproliferation in guinea pigs.29 Clinical studies corroborated the findings in animals. The regular intake of GLA-rich vegetable oils has been shown to normalize the balance of inflammatory eicosanoids and to mitigate the symptoms of chronic inflammatory disorders such as rheumatoid arthritis and atopic eczema.32,33 Topically or systemically applied evening primrose or borage oil has been shown to help restore the natural epidermal barrier function or accelerated its recovery when it had been previously injured.2-4, 34, 35 TEWL was lowered, skin moisture increased and the skin attained a smoother appearance.
The present study shows that the regular intake of evening primrose oil, oil with a high GLA content, demonstrably strengthens the physiological skin barrier function, improves the biomechanical properties and smoothes the surface profile of irritated skin. Supporting the conclusion is the observation that it took longer than four weeks for the effects to show, which is in line with the turnover time of four weeks for middle-aged skin and with the time for fatty acids to reach new steady-state equilibrium. Even though the exact mechanism of activity is unknown, this study underpins the importance of preformed GLA for healthy skin.
According to the latest market trends, beauty supplements is a rapidly growing category. Evening primrose, with its demonstrated efficacy to optimize skin health and help fight visible signs of aging after systemic application, thus is an ideal candidate. The oil has a long history of safe use as an ingredient in foods, dietary supplements and topical cosmetics; as a vegetable oil it additionally could qualify as an ingredient for natural, herbal or organic cosmetic lines.
References
1. Mac-Mary, JM Sainthillier and P Humbert, Dry skin and the environment, Exog Dermatol 3 72–80 (2004)
2. HP Nissen, H Biltz and R Muggli, Borage oil, gamma-linolenic acid decreases skin roughness and TEWL and increases skin moisture in normal and irritated human skin, Cosm & Toil 110 71–74 (1995)
3. T Brosche and D Platt, Effect of borage oil consumption on fatty acid metabolism, transepidermal water loss and skin parameters in elderly people, Arch Gerontol Geriatr
30 139–150 (2000)
4. R Muggli, Systemic evening primrose oil improves the biophysical skin parameters of healthy adults, Int J Cosmet Sci 27 243–249 (2005)
5. J Bettinger et al, Comparison of different non-invasive test methods with respect to the effects of different moisturizers on skin,
Skin Res Technol 5 21–27 (1999)
6. J Pinnagoda, RA Tupker, T Agner and J Serup, Guidelines for transepidermal water loss (TEWL) measurement, A report from the Standardization Group of the European Society of Contact Dermatitis, Contact Derm 22 164–178 (1990)
7. A Fullerton et al, Guidelines for measurement of skin color and erythema, A report from the Standardization Group of the European Society of Contact Dermatitis, Contact Dermatitis 35 1–10 (1996)
8. AO Barel, W Courage and P Clarys, Suction method for measurement of skin mechanical properties: The Cutometer in Handbook of Non-invasive Methods and the Skin, J Serup and GBE Jemec, eds, Ann Arbor: CRC Press 335–340 (1995)
9. S Jaspers et al, Rapid in vivo measurement of the topography of human skin by active image triangulation using a digital micromirror device, Skin Res Technol 5 195–207 (1999)
10. HS Hansen and B Jensen, Essential function of linoleic acid esterified in acylglucosylceramide and acylceramide in maintaining the epidermal water permeability barrier, Evidence from feeding studies with oleate, linoleate, arachidonate, columbinate and alpha-linolenate, Biochim Biophys Acta 834 357–363 (1985)
11. PW Wertz, DC Swartzendruber, W Abraham, KC Madison and DT Downing, Essential fatty acids and epidermal integrity, Arch Dermatol 123 1381–1384 (1987)
12. VA Ziboh, CC Miller and Y Cho, Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: Generation of anti-inflammatory and antiproliferative metabolites, Am J Clin Nutr 71 (suppl) 361S–366S (2000)
13. P DeLuca, RG Rossetti, C Alavian, P Karim and RB Zurier, Effects of gammalinolenic acid on interleukin-1β and tumor necrosis factor-α secretion by stimulated peripheral blood monocytes: Studies in vitro and in vivo, J Investig Med 47 246–250 (1999)
14. RK Furse, RG Rossetti, CM Seiler and RB Zurier, Oral administration of gamma linolenic acid, an unsaturated fatty acid with anti-inflammatory properties, modulates interleukin-1β production by human monocytes, J Clin Immunol 22 83–91 (2002)
15. MBW Dooper, B van Riel, YMF Graus and L M’Rabet, Dihomo-γ-linolenic acid inhibits tumour necrosis factor-α production by human leucocytes independently of cyclooxygenase activity, Immunol 110 348–357 (2003)
16. DF Horrobin, Gamma-linolenic acid: An intermediate in essential fatty acid metabolism with potential as an ethical pharmaceutical and as a food, Rev Contemp Pharmacother 1 1–45 (1990)
17. DB Jones, RD Carter and JI Mann, Indirect evidence of impairment of platelet desaturase enzymes in diabetes mellitus, Horm Metab Res 18 341–244 (1986)
18. DC Leng, FB Smith, FG Fowkes et al, Relationship between plasma essential fatty acids and smoking, serum lipids, blood pressure and homeostatic and rheological factors, Prostaglandins Leukot Essent Fatty Acids 51 101–108 (1994)
19. LC Wood et al, Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice, J Clin Invest 90 482–487 (1992)
20. LC Wood et al, Barrier disruption stimulates interleukin-1α expression and release from a pre-formed pool in murine epidermis, J Invest Dermatol 106 397–403 (1996)
21. JC Ansel et al, The expression and modulation of IL-1 alpha in murine keratinocytes, J Immunol 140 2274–2278 (1988)
22. D Black, A Del Pozo, JM Lagarde and Y Gall, Seasonal variability in the biophysical properties of stratum corneum from different anatomical sites, Skin Res Technol 6 70–76 (2000)
23. M Denda et al, Low humidity stimulates epidermal DNA synthesis and amplifies the hyperproliferative response to barrier disruption; implication for seasonal exacerbations of inflammatory dermatoses, J Invest Dematol 111 873–878 (1998)
24. Y Ashida, M Ogo and M Denda, Epidermal interleukin-1α generation is amplified at low humidity: Implications for the pathogenesis of inflammatory dermatoses, Br J Dermatol 144 238–243 (2001)
25. RS Chapkin, VA Ziboh and JL McCullough, Dietary influences of evening primrose and fish oil on the skin of essential fatty acid-deficient guinea pigs, J Nutr 117 1360–1370 (1987)
26. CC Miller, W Tang, VA Ziboh and MP Fletcher, Dietary supplementation with ethyl ester concentrates of fish oil (n-3) and borage oil (n-6) polyunsaturated fatty acids induces epidermal generation of local putative anti-inflammatory metabolites, J Invest Dermatol 96, 98–103 (1991)
27. PJ Hartop and C Prottey, Changes in transepidermal water loss and the composition of epidermal lecithin after application of pure fatty acid triglycerides to the skin of essential fatty acid-deficient rats, Br J Dermatol 95 255–264 (1976)
28. CC Miller and VA Ziboh, Gammalinolenic acid-enriched diet alters cutaneous eicosanoids, Biochem Biophys Res Commun 154 967–974 (1988)
29. S Chung, S Kong, K Seong and Y Cho, γ-Linolenic acid in borage oil reverses epidermal hyperproliferation in guinea pigs, J Nutr 132 3090–3097 (2002)
30. VA Ziboh and RS Chapkin, Biologic significance of polyunsaturated fatty acids in the skin, Arc, Dermatol 123 1686a–1690a (1987)
31. C Prottey, PJ Hartop, JG Black and JI McCormack, The repair of impaired epidermal barrier function in rats by the cutaneous application of linoleic acid, Br J Dermatol 94 13-21 (1976)
32. JJF Belch and A Hill, Evening primrose oil and borage oil in rheumatologic conditions, Am J Clin Nutr 71 (suppl) 352S-356S (2000)
33. DF Horrobin, Essential fatty acid metabolism and its modification in atopic eczema, Am J Clin Nutr 71 (suppl) 367S–372S (2000)
34. W Gehring, R Bopp, F Rippke and M Gloor, Effect of topically applied evening primrose oil on epidermal barrier function in atopic dermatitis as a function of vehicle, Arzneimittelforschung 49 635–642 (1999)
35. HP Nissen, W Wehrmann, U Kroll and HW Kreysel, Influence of polyunsaturated fatty acids on the plasma phospholipids of atopic patients, Fat Sci Technol 7 268–271 (1988)