
Over the years, the cosmetics and personal care industry has become more complex. In addition to chemistry, biochemistry, biophysics and microbiology have become very important to developing products to meet consumer needs.
Since not all formulators have the same background, terms like fatty acid, triglyceride, amino acid, hydroxy acid and the like dominate our discussions, and I believe can sometimes get blurred in common use. This installment of "Comparatively Speaking" aims to clarify them.
Amino Acids
Amino acids are organic compounds that have both an amino group and a carboxyl group present in the same molecule. They have the structure shown in Figure 1. Amino acids are the building blocks (monomers) that link together to form proteins. Proteins perform many functions in our bodies. One major protein class is enzymes, and these are necessary for most of our metabolism.
Figure 1. Amino Acid Structure1
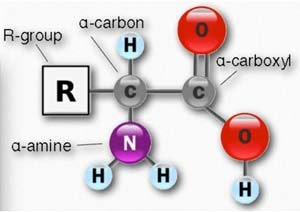
Amino acids in proteins make up the second-highest percentage of human weight, exceeded only by water. Amino acids are formed by the reaction of the amino group of one amino acid molecule and the carboxyl group of another (see Table 1). The resulting compound, called an amide in organic chemistry, is referred to as a peptide.
Table 1. Structure of Amino Acids2
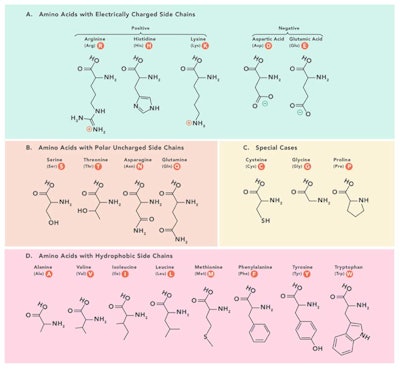
The nature of the R group (see Figure 1) controls how the protein folds and consequently, how the protein functions. Biological protein synthesis is controlled by DNA and is made by a complex set of reactions.
Fatty Acids
Fatty acids are a class of compounds that are commonly derived from triglycerides. Generally, a fatty acid consists of a straight chain of an even number of carbon atoms, with hydrogen atoms along the length of the chain and at one end of it, and a carboxyl group at the other end.3 The structure is: R-C(O)-OH.
Triglycerides, also called triacylglycerols, are the triesters of glycerin with three equivalents of fatty acid. Fatty acids are defined as those acids having alkyl or alkylene groups being C-5 and higher. When the triglyceride is saponified, fatty acids and glycerin are liberated. Saponification is a general term to define the chemical reaction that breaks the ester linkage. The saponification reaction is shown in Figure 2.
Figure 2. Saponification Reaction4
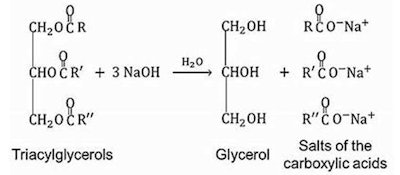
Triglycerides are digested by the body to provide energy for metabolism, or are stored by the body when there is excess available.
Triglycerides are the most common form of fat in the body and they are the main ingredient in vegetable oils and animal fats. The fatty acid compositions of some common edible fats and oils are shown in Tables 2 and 3.
Table 2. Fatty Acid Compositions of Common Edible Fats and Oils5
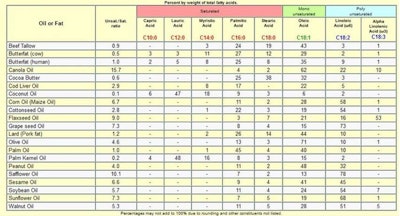
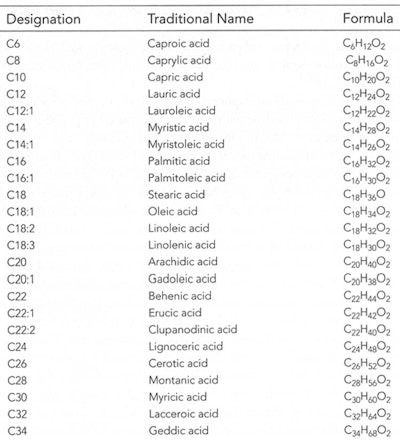
Table 3. Common Fatty Acids6
References
1. https://biochem.oregonstate.edu/content/biochemistry-free-and-easy
3. Encyclopedia Britannica (2020, May 12). Fatty acid. Available at https://www.britannica.com/science/fatty-acid
4. https://www.hielscher.com/fr/ultrasonic-saponification.htm
5. https://i.pinimg.com/736x/c8/f4/34/c8f434c95e93b5253ec2800037987a94.jpg
6. https://www.scientificspectator.com/documents/book%20service/Oils_of_Nature.pdf










