What happens to certain skin care active ingredients after they are applied to the skin and exposed to sunlight? Do they function as intended? Or do they undergo chemical or structural changes that render them less effective or even ineffective?
Previously, the skin care active ingredients retinol (vitamin A) and retinyl palmitate were studied by these authors, finding that exposure to UVR caused them to lose their structural and, to some extent, their chemical integrity.1 In this article, similar stability studies on trans-resveratrol are reported.
Resveratrol is a polyphenol that occurs naturally in at least 72 plant species.2 Dietary sources include peanuts, peanut butter, the seeds and skins of grapes and wine. Resveratrol is a member of the stilbene family of chemicals, whose members exhibit two aromatic rings connected by a methylene bridge. Resveratrol exists in two isomeric forms, trans- and cis- (see Figure 1) and as the glucoside piceid, also called polydatin. The trans- isomer is the more common and is believed to be more stable and biologically active.3
Resveratrol is an antioxidant, a compound that inhibits or counteracts an oxidizing agent. Oxidizing agents include free radicals, which are atoms or molecules with an unpaired electron. Examples of free radicals include superoxide anion (O2), peroxyl radical (COO˙) and hydroxyl radical (˙OH). Other highly reactive oxidizing agents include molecular oxygen (O2), singlet oxygen and hydrogen peroxide (H2O2).4 Oxidizing agents that contain oxygen are referred to as reactive oxygen species (ROS). Each is capable of initiating and propagating a free radical “chain reaction” with potentially damaging consequences for cellular membranes, proteins and DNA.5
Resveratrol and its isomers are of great interest to personal care formulators because they offer a wide range of benefits. Soares, Andreazza and Salvador found resveratrol to be superior to propyl gallate, vitamin C and vitamin E in its ability to sequester 2,2'-azinobis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) and 1,1-diphenyl-2-picrylhydrazyl (DPPH), and in its ability to scavenge hydroxyl radicals.6 Aziz et al. and Jang et al. found that topical application of resveratrol inhibits UVB-induced tumor initiation, promotion and progression in skin.7, 8 Liu et al. determined that resveratrol inhibits human lung adenocarcinoma cell metastasis.9 Resveratrol has also demonstrated antimicrobial activity toward Propionibacterium acnes and anti-inflammatory properties, leading to speculation that it may be a therapeutic option for treating acne vulgaris.10
Of the two naturally occurring stereoisomers (see Figure 1), the trans- isomer is more photo- and thermally stable. Several studies have documented the effects of exposing trans-resveratrol to UVR. For example, Montsko et al. irradiated trans-resveratrol in ethanol at 365 nm and found that, in addition to the cis- isomer, a second photoproduct appeared with increasing concentration as irradiation continued up to one hour. This additional photoproduct was tentatively identified by mass spectrometry to be a diphenyl-acetylene derivative of trans-resveratrol resulting from oxidation of the central double bond to a triple bond.11
In 2010, companies launched 62 skin care products containing trans-resveratrol.12 Given the well-documented photolability of resveratrol, it seemed appropriate to consider how best to preserve trans-resveratrol’s chemical form and, presumably, its efficacy after a topical composition is applied and exposed to sunlight. Ethylhexyl methoxycrylene (EHMC) was selected as a candidate to photostabilize trans-resveratrol because of its proven efficacy as a photostabilizer of both UV filters, notably avobenzone, and the skin care active ingredients retinol and retinyl palmitate.1, 13–14
Materials and Methods
Trans-resveratrol ≥ 99% was purchaseda and also provided as a free sampleb, and EHMC was also suppliedc. Solvents used in high-performance liquid chromatography (HPLC) studies were HPLC-grade. Other ingredients used to prepare test compositions were obtained as free samples from well-known cosmetic raw material suppliers.
UV absorbance measurements: The UV absorbance spectra of trans- and cis-resveratrol were determined at 10 ppm in acetonitrile (AcCN) on a spectrophotometerd. The UV absorbance spectra of the trans- and cis-isomers of resveratrol were recorded by the HPLC UV detectore and identified with corresponding peaks as they eluted.
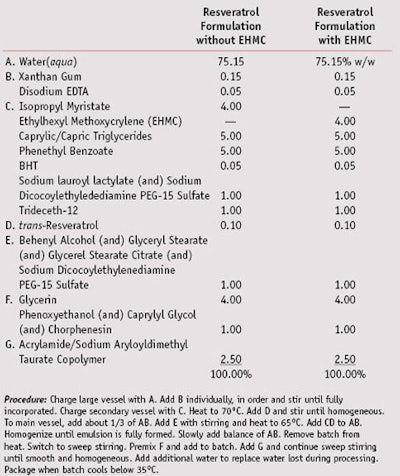
Preparation of test formulations: Test formulations were prepared as o/w emulsions. The formulas and preparative procedures can be found in Table 1.
UV exposure studies: Approximately 0.15 g of each test formulation was applied to a 5 cm x 2.5 cm quartz slide and covered with another quartz slide of the same size. Light pressure was applied to spread the test formulation evenly between the two slides. Slides were irradiated with 5 minimum erythemal doses (MED) of full spectrum UVR (290–400 nm) at three different locations. (1 MED = 21 mJ/cm2. Five MED is roughly equivalent to one hour exposure of midday, midsummer sun). A solar simulator equipped with a UVB detector and microprocessor-based controllerf provided the UV radiation. The xenon lamp’s output in the solar simulator was filtered to remove wavelengths <290 nm, i.e., UVC, and >400 nm, i.e., visible light and infrared radiation.
Following irradiation, residual sample material was extracted from the slides with AcCN and the resulting mixtures were mixed well and filtered through polytetraflouroethylene (PTFE) filters before being subjected to HPLC analysis. Non-irradiated samples were processed in an identical manner. Phenoxyethanol, part of the preservative system, was used as the internal standard for calculating resveratrol content.
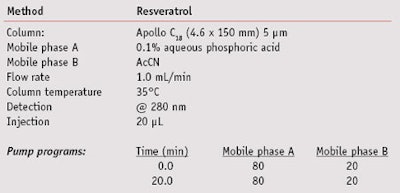
High-performance liquid chromatography: Chromatographic analyses of 0.1% resveratrol formulations before and after irradiation were performed on an HPLC systemg equipped with quaternary pumps, a vacuum degasser, an auto-injector and a dynamic absorbance detector (DAD), all connected to a computer running specialized softwareh. The reagents, AcCN and 0.1% phosphoric acid in water, were both HPLC-grade. Chromatographic separation was achieved using an C18 columni under the conditions summarized in Table 2.
Testing oxidative and thermal degradation: A thin layer of 0.2 g of a composition containing 0.1% trans-resveratrol and no EHMC, shown in Table 1, was spread on the inside bottom of a 100 mL glass beaker, which was then covered completely with aluminum foil to protect the contents from light. The covered beaker was then placed in a 400 mL beaker, which was set in a water bath equilibrated to 37C and incubated for a predetermined time. After incubation, the 0.1% trans-resveratrol composition was extracted with AcCN, filtered through a PTFE filter, and analyzed by HPLC. The experiment was repeated to determine trans-resveratrol concentration after 0, 2, 4 and 16 hr of incubation.
DPPH antioxidant studies: Trans-resveratrol’s antioxidant activity before and after irradiation with UV was determined by measuring spectrophotometrically the reduction of the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH).
A 0.005 % w/w trans-resveratrol solution was prepared in methanol. One aliquot of the 0.005% trans-resveratrol solution was kept in the dark; the other aliquot was irradiated with 20 MED in a quartz cuvette. The following procedure was used to determine antioxidant activity of the two solutions:
2.5 mL of methanolic DPPH solution at 100 micromole/L concentration was placed in a quartz cuvette and 1 mL of the 0.005% [w/w] solution of trans-resveratrol was added. The UV absorption of the remaining DPPH was measured on a spectrophotometerd at 30-second intervals until the decrease in UV absorption was less than 2% per hour.
Testing fluorescence quenching: Qualitative fluorescence quenching experiments were performed by preparing dilute solutions (0.2% w/w) of trans-resveratrol in ethyl acetate. Three solutions contained trans-resveratrol alone or combined with 1% and 2% of a non-photoactive diluent (phenethyl benzoate), which served as negative controls. Two additional solutions were prepared with 1% and 2% EHMC in place of the phenethyl benzoate, which served as the test solutions. Fifteen microliters each of the control and test solutions was spotted on thin layer chromatography (TLC) platesj using a micropipette and allowed to dry. The TLC plates were then illuminated with long-wave UV radiationk and a photograph of the illuminated plate was takenm.15
Results
Figure 2 depicts the UV absorbance spectra of trans-resveratrol and cis-resveratrol as recorded by the DAD of the HPLC system. The UV absorption of trans-resveratrol was also measured at 10 ppm in AcCN on a spectrophotometerd. Its peak absorbance was measured at 1.296 at 304 nm and 1.335 at 321 nm. The molar mass of resveratrol is 228.24 grams. Therefore, its molar extinction coefficient (ε) is 29,580 at 304 nm and 30,470 at 321 nm. This result agrees with the molar extinction coefficient of 30,335 at 304 nm in water reported by Camont et al.16
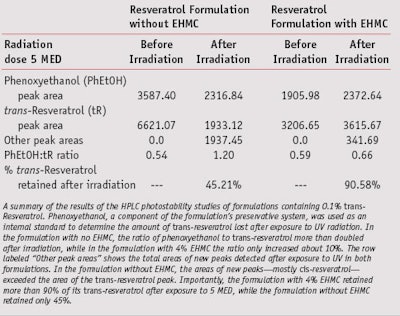
Figures 3, 4 and 5 are the relevant portions of the HPLC chromatograms recorded before irradiation with no EHMC, after 5 MED with no EHMC, and after 5 MED with 4% EHMC, respectively. The chromatograms show the peaks for trans-resveratrol in blue and cis-resveratrol in green. The amount of trans-resveratrol in each experiment was quantified by dividing the area of the phenoxyethanol peak by the area of the trans-resveratrol peak as determined at 280 nm by the DAD. Table 3 summarizes the areas of each peak and their ratios in the three experiments.
Table 4 shows that the percent of trans-resveratrol remaining by HPLC after being spread in a thin layer on glass and incubated at 37C for 2, 4, and 16 hours was 99.30%, 99.93% and 98.79%, respectively.
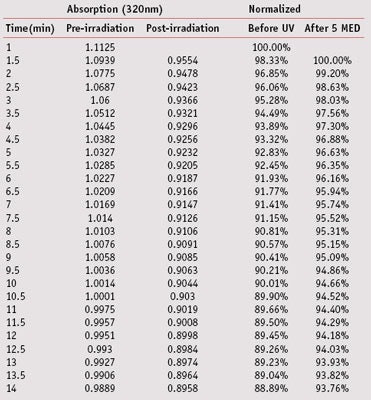
Table 5 presents the raw data collected from the DPPH assay of antioxidant activity of a 0.005% methanolic solution of trans-resveratrol before and after irradiation with 5 MED. The two columns on the right of the table present the data after being normalized to depict the absorption of the DPPH solutions at 320 nm as a percentage of their absorption close to the starting time. Figure 6 presents the data graphically. The decline in absorption is significantly greater in the non-irradiated solution as evidenced by the relatively steeper slope compared to the irradiated solution.
Figure 7 is a photograph of the qualitative experiment to determine whether or not EHMC has a quenching (extinguishing) effect on the visible fluorescence of trans-resveratrol. Test spots are on the top row; control spots are on the bottom. All six spots were made by applying 15 µl of a 0.2% solution of trans-resveratrol in ethyl acetate to a thin layer chromatography plate and, after drying, illuminating the plate with long wave UV radiation. The top left, top middle and top right spots contain 0%, 1% and 2% EHMC, respectively. Fluorescence quenching is strongly supported if, as shown, the top middle spot is darker than the top left, and the top right spot is darker than the top left. It appears in Figure 7 that the top right spot with 2% EHMC shows the complete absence of fluorescence.
Discussion
The high extinction coefficient of trans-resveratrol at 304 nm and 320 nm indicates its relative high efficiency in converting photons between those wavelengths into electronic excitation energy. For comparison, the popular UV filter ethylhexyl methoxycinnamate (OMC) has a molar extinction coefficient of about 25,000. In other words, trans-resveratrol is a good UV absorber.
Trans-resveratrol is a fluorescent compound, meaning that a photonically excited molecule of trans-resveratrol is capable of deactivation from the singlet excited state by emitting a photon. Its fluorescence emissions peak at 385 nm and have a significant tail past 400 nm into the visible range.17 The left-most spot on the top row of Figure 7 and the three spots on the bottom row show the visible fluorescence of a 0.2% solution of trans-resveratrol in ethyl acetate applied to a TLC plate, allowed to dry and illuminated by long-wave UV.
As Camont, Montsko and others have reported, and as this report confirms, the efficiency of trans-resveratrol in absorbing UVR results in a high rate of conversion of the trans- isomer to the cis-. However, in the presence of the photostabilizer EHMC, UV-induced trans-cis isomerization was greatly reduced. More than 90% of the original concentration of trans- isomer was preserved. Figure 7 shows that the addition of 1% EHMC (top middle spot) and 2% EHMC (top right spot) to the 0.2% trans-resveratrol solution dramatically reduces or eliminates the intensity of the fluorescence in a concentration-related manner. While this result is not definitive and does not rule out a Dexter exchange (triplet) mechanism, it provides strong evidence that singlet energy transfer is an important mechanism by which EHMC inhibits resveratrol’s UV-induced trans-cis isomerization.
Stilbenes such as resveratrol are known to undergo electrocyclic reactions to a dihydrophenanthrene, which proceed only from the cis- isomer.18 This study documents the appearance of one or more photoproducts, which eluted about two minutes after the trans- isomer and about one minute after the cis-. The identity of the photoproducts in this study remains undetermined. Regardless, it is clear in this study and others that exposure to UV invariably leads to a large and rapid reduction in the population of the trans- isomer. The cis- isomer of resveratrol is so thermally and oxidatively unstable that it is not commercially available. For that reason, most studies of resveratrol’s biological activity have used the trans- isomer and little is known about the biological activity of the cis- isomer.
In this paper, antioxidant activity serves as a proxy for some or many of trans-resveratrol’s biological activities. The goal was to get a partial answer to the question: Is UV-induced photoisomerization and photodegradation of trans-resveratrol biologically relevant? Logically, if antioxidant activity is not reduced by UV exposure, the answer would be no, at least as far as antioxidant activity is concerned. However, the answer found in the DPPH assay is yes (see Figure 7): UV irradiation does reduce the antioxidant activity of trans-resveratrol. So at least in a limited sense, the consequences of UV exposure are biologically relevant.
A 5 MED radiation dose was used for both the HPLC photostability studies and the DPPH antioxidant study. However, trans-resveratrol’s photostability was measured in emulsified compositions, while its antioxidant activity was measured in solution. The differences in the two experimental models make correlating the results between them difficult. Still, it appears to be more than a coincidence that, after irradiation with 5 MED, a loss of trans- isomer was observed in one study and a decline in antioxidant activity was recorded in the other.
Concern about trans-resveratrol’s oxidative and thermal stability has grown as its use as a dietary supplement and topical active ingredient has grown. Prokup et al. investigated the long-term stability of trans-resveratrol and piceid, the glucoside of trans-resveratrol, in powder form. The neat compounds were analyzed by HPLC after being held at 75% humidity and 40C for four years without detecting any instability.19 The study reported in this article also showed no significant instability, at least on the timescales associated with normal application to the skin. Granted, the 0.1% trans-resveratrol composition was applied to glass, not skin, before incubation at 37C. Perhaps in the future this experiment will be repeated using a cultured skin model or ex vivo human skin.
Conclusions
These authors have reported on the photoinstability of trans-resveratrol, confirming other research that UV irradiation causes trans-resveratrol to undergo a rapid isomerization from the more stable trans- isomer to the less stable cis- isomer, and the production of as-yet-unidentified photoproducts. It has been shown that the UV-induced changes probably indicate a reduction in trans-resveratrol’s antioxidant activity as demonstrated by its decreased ability to reduce the stable free radical DPPH after UV exposure. And it has been shown that the sunscreen photostabilizer ethylhexyl methoxycrylene strongly inhibits UV-induced changes to trans-resveratrol and probably does so partially by a singlet quenching mechanism.
It has been demonstrated that trans-resveratrol degradation and decomposition is solely a consequence of UV exposure and not due to oxidative and thermal mechanisms, at least on the timescales associated with normal use as a topical medicament.
Formulators who want their trans-resveratrol products to deliver biological benefits should include a proven photostabilizer to preserve the structural integrity of trans-resveratrol (see Figure 8). Ethylhexyl methoxycrylene is a good choice to accomplish that goal.
References
Send e-mail to [email protected].
1. CA Bonda and J Zhang, Photostabilization of Retinol and Retinyl Palmitate by Ethylhexyl Methoxycrylene, Cosm & Toil 126(1) 40–48 (2011)
2. W. Dercks and LL Creasy, Influence of fosetyl-A1 on phytoalexin accumulation in the Plasmopara viticola–grapevine interaction, Physiol Mol Plant Pathol 34 203–213 (1989)
3. RE King, JA Bomser and DB Min, Bioactivity of Resveratrol, Comprehensive Reviews in Food Science and Food Safety 5 65–70 (2006)
4. SR Pinnel, Cutaneous photodamage, oxidative stress, and topical antioxidant protection, J Am Acad Dermatol 48(1) 1–19 (2003)
5. AJ Levine and AM Puzio-Kuter, The Control of the Metabolic Switch in Cancers by Oncogenes and Tumor Suppressor Genes, Science 330 1340–1344 (Dec 2010)
6. DG Soares, AC Andreazza and M Salvado, Sequestering ability of butylated hydroxytoluene, propyl gallate, resveratrol, and vitamins C and E against ABTS, DPPH, and hydroxyl free radicals in chemical and biological systems, J Agric Food Chem 51 1077 (2003)
7. MH Aziz, R Kumar and N Ahmad, Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (Review), Int J Oncol 23 17–28 (2003)
8. M Jang, L Cai, GO Udeani, et al, Cancer chemoprevention activity by resveratrol, a natural product derived from grapes, Science 275 218–220 (1997)
9. P-L Liu, J-R Tsai, AL Charles, J-J Hwang, S-H Chou, Y-H Ping, F-Y Lin, Y-L Chem, C-Y Hung, W-C Chen, Y-H Chen and I-W Chong, Resveratrol inhibits human lung adenocarcinoma cell metastasis by suppressing heme oxygenase 1-mediated muclear factor-κB pathway and subsequently downregulating expression of matrix metalloproteinases, Mol Nutr & Food Res 54(S2) S196–S204 (Jul 2010)
10. E Taylor, J Champer and J Kim, In vitro activity of Resveratrol against Propionibacterium acnes, J Am Acad Dermatol, poster presentation in Miami, Fla., USA (Mar 2010)
11. G Montsko, MS Pour Nikfardjam, Z Szabo, K Boddi, T Lorand, R Ohmacht and L Mark, Determination of products derived from trans-resveratrol UV photoisomerization by means of HPCL—APCI-MS, J Photochem Photobiol A: Chemistry 196 44–50 (2008)
12. Mintel Research (2011)
13. CA Bonda, A Pavlovic, K Hanson and C Bardeen, Singlet Quenching Proves Faster is Better for Photostability, Cosm & Toil 125(2) 40–48 (2010)
14. Sunscreen Photostability Course
15. US Patent 7,776,614, Test method for determining compounds capable of quenching electronic singlet state excitation of photoactive compounds, C Bonda, assigned to the HallStar Company (Aug 17, 2010)
16. L Camont, C-H Cottart, Y Rhayem, V Nivet-Antoine, R Djelidi, F Collin, J-L Beaudeux and D Ronnefont-Rousselot, Simple spectrophotometric assessment of the trans-/cis-resveratrol ratio in aqueous solutions, Analytica Chemica Acta 634 121–128 (2009)
17. YL Jiang, Design, synthesis, and spectroscopic studies of Resveratrol aliphatic acid ligands of human serum albumin, Bioorg Medicinal Chem 16 6406–6414 (2008)
18. NJ Turro, Modern Molecular Photochemistry, Benjamin/Cummings, Menlo Park, Calif., 1978 pp 503–504
19. J Prokop, P Abrman, A Seligson and M Sovak, Resveratrol and its Glycon Piceid Are Stable Polyphenols, Journ Medicinal Food 9(1) 11–14 (2006)