
DoE methods examined component interactions in bigels to optimize sensoriality, performance and stability. Tests using HEC and tucuma oil revealed effects on mechanics, rheology and stability, underscoring the need to balance internal/external phases.
This article is only available to registered users.
Log In to View the Full Article
DoE methods examined component interactions in bigels to optimize sensoriality, performance and stability. Tests using HEC and tucuma oil revealed effects on mechanics, rheology and stability, underscoring the need to balance internal/external phases.
Dispersed or colloidal systems, such as foams, emulsions and gels, involve the dispersion of particles within a continuous phase.1 Gels, in particular, are formed by dispersing a solid (gelling agent) in a liquid, creating a three-dimensional network that traps the liquid phase. Based on the nature of the liquid phase, gels can be classified into hydrogels and organogels,2 or their combination (bigels).
The present work details the properties and dynamics of these gel types. It also evaluates the influence of various components on the physicochemical characteristics of bigels by using tucuma oil and hydroxyethylcellulose, and applying a design of experiments (DOE) approach to gain a deeper understanding of their interactions to optimize their performance for future applications.
Hydrogels
Hydrogels, which are prepared using natural or synthetic hydrophilic polymers, have well-established uses in drug delivery systems, cell culture media and wound healing dressings due to their ease of preparation and availability. These systems, due to their high water content, offer numerous advantages for various biomedical and cosmetic applications. Hydrogels are well-suited for applications such as topical drug delivery, as they can increase skin hydration and maintain moisture on the skin surface.
Organogels
In contrast, organogels have gained growing interest for their inherent long-term stability. The colloidal network in organogels is formed by polymeric organogelators that cross-link or entangle, creating a network structure. This network can be strengthened by low molecular weight (LMW) organogelators, which form gels with crystalline fibers or particles that create a cluster-based network.
This structure exhibits plastic behavior due to weak surface interactions, which has been observed in colloidal silica organogels where hydrogen bonds on the silica surface facilitate interactions.3 Pharmaceutical organogels are often produced with biocompatible solvents and natural vegetable oils, which serve as emollients that increase skin hydration and prevent water loss from the stratum corneum.4 These oils are known for their ability to repair and restore the skin barrier, providing a smooth and moisturizing effect on the skin.
Biphasic Gels
Biphasic gels, or bigels, are used in a variety of commercial cosmetic products, particularly in facial serums and sunscreens, where their unique texture and dual-phase properties enhance sensory appeal and efficacy. They are created by mixing hydrogels and organogels in various proportions, maintaining the semisolid structure of both phases.5-8 These systems were introduced to address some of the limitations of oleogels, such as difficulty in removal after application, which can negatively impact patient compliance.9
Moreover, bigels exhibit enhanced drug release rates compared to organogels, thanks to the combined properties of both oleogels and hydrogels. These properties include increased water content in the stratum corneum, which facilitates better absorption and hydration; easy removal with water; a cooling effect; and good spreadability. These attributes make bigels particularly promising for cosmetic and pharmaceutical applications, especially for skin treatments and drug delivery.8, 10
Structure and Function Modulation
Adjusting the oleogel-to-hydrogel volume ratio and the hydrogel matrix composition can modulate the microstructure and functional properties of bigels. This flexibility enables the tailoring of bigels for given applications; e.g., optimizing their stability, flavor retention and controlled release behavior.
More specifically, the transition from oleogel-in-hydrogel to a bicontinuous structure allows for enhanced control over freeze-thaw stability, flavor sensation during oral consumption, and the release profiles of both hydrophilic and lipophilic compounds, offering a significant advantage in formulation design.11 Studies also have shown that altering the oleogel-to-hydrogel ratio can control texture and rheological properties to customize sensory characteristics.12
Recent research has further demonstrated that lipid selection and excipient compatibility are crucial factors in formulation stability and that they directly influence the physical properties of bigels, such as viscosity, spreadability and drug release rates. Moreover, many-objective optimization techniques have proven effective in balancing conflicting parameters such as droplet size, drug release and stability, facilitating the development of high-performance bigel systems for specific therapeutic and cosmetic purposes.13
Additionally, the application of statistical approaches such as the Box-Behnken design and the Quality by Design methodology has proven valuable to optimize bigel formulations; for example, the development of a metronidazole-loaded bigel has been demonstrated, resulting in a more precise and efficient formulation process.23
Tucuma Oil
Various vegetable oils, including olive, almond, sunflower and sesame, have been used in the development of bigels. In the present work, tucuma oil, derived from the fruits of Astrocaryum vulgare, a palm tree native to tropical South American ecosystems such as the Amazon rainforest, was chosen for its unique properties.14
Tucuma oil is rich in palmitic (30.4%) and oleic (59.9%) acids, along with a high concentration of beta-carotene, making it a potent antioxidant and anti-inflammatory agent.15, 16 As such, tucuma oil has been investigated for antihyperglycemic, antimicrobial and antioxidant applications.16-18 This makes tucuma oil an ideal candidate for use in bigel formulations, as it can enhance both the physical stability and therapeutic effectiveness of the system.
Study Rationale
Several studies have explored the influence of different components on the physicochemical characteristics of bigels, with some authors emphasizing the impact of the inner phase and others focusing on the outer phase.2, 5, 7-9, 19 Despite this, efforts to model bigels based on rheological behavior have revealed that existing methods do not fully capture the complexity of bigel characteristics.16
Therefore, as stated, this study aims to evaluate the influence of various components on the physicochemical characteristics of bigels using a DoE approach to gain a deeper understanding of their interactions and optimize their performance for future applications. Tucuma oil and hydroxyethylcellulose served as test components for the following experiments.
Materials and Methods
Materials: Phenoxyethanol and paraben preservative, vitamin E, propylene glycol and hydroxyethylcellulose were purchased for the described experimentsa while the tucuma oil was obtained separatelyb and the stabilizer/emulsifier was kindly donatedc.
Bigel preparation: Hydrogels and organogels were prepared separately using different procedures depending on the composition. All samples were stored in opaque polyethylene flasks at room temperature after preparation.
Hydrogel: Hydrogels were prepared by completely dissolving methylparaben and propylparaben in a sufficient amount of water at 70ºC. Carboxymethylcellulose (CMC) or hydroxyethylcellulose (HEC) was then dispersed under continuous stirring until a firm and transparent hydrogel was obtained. Propylene glycol (PPG) was added before dispersing HEC when it was used.
Tucuma butter organogels: Tucuma butter (TB) was melted at 60ºC in a water bath and BHT was dissolved. The stabilizer/emulsifier was then added and gently stirred until complete dispersion.
Tucuma oil organogels: Tucuma oil (TO) organogels were obtained at room temperature by dissolving BHT and dispersing the stabilizer/emulsifier in TO under slight stirring.
Bigels: Bigels were prepared differently depending on the organogel type. TB bigels were obtained by heating both aqueous and oil phases at 70ºC. The oil phase was then poured onto the aqueous phase and homogenizedd with a high shear mixer at 10,000 rpm. Oil bigels were prepared at room temperature by mixing aqueous and oil phases also using a high shear mixer at 10,000 rpm.
Bigel Characterization and Properties
Characterization: Bigel microstructures were observed using bright field optical microscopye at a magnification of 40x to analyze phase interaction and morphology. Thermal properties of bigels, hydrogels and organogels were analyzed using thermogravimetric analysisf.
Samples were heated from 50ºC to 600ºC at a rate of 10ºC/min under a nitrogen atmosphere with a flow rate of 25 mL/min. The pH of bigel samples was measured after 24 hr of production at 24ºC using a digital pH meterg. All samples were diluted to 10% (w/v) using purified water as the solvent.
Spreadability: Spreadability was evaluated using a simple plate method. A circular acrylic plate with a diameter of 10 cm and a thickness of 0.2 cm, featuring a 1-cm diameter hole, was used to standardize the initial diameter and volume of the samples. Measurements were conducted using a millimeter base.
Samples were placed into the circular plate hole and their surfaces were leveled. After removing the circular plate, a sequence of glass plates with known weights were placed onto the sample at intervals of 60 sec. The medium diameter of the sample was recorded before adding each plate. Sample limit spreadability was determined at 25 ºC and calculated using Equation 1:
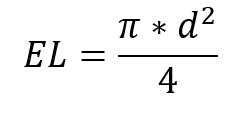 Equation 1
Equation 1
where EL is the limit spreadability for the plate with constant medium diameter (mm2) and d is the constant medium diameter (mm). The spreadability of samples was then calculated according to the added glass plates weight according to the Equation 2:
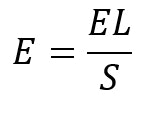 Equation 2
Equation 2
where E is the sample spreadability (mm2/g), EL is the limit spreadability (mm2) and S is the weight (g) of the added glass plates.
Stability: For the stability study, all bigel samples were centrifuged after 24 hr of preparation for 40 min at 2,500 rpm without temperature control. Phase separation was visually observed and recorded.
Statistical analysis: Bigel samples were prepared according to a randomized experimental design in a factorial model 3x3, varying HEC concentrations (2.0%, 2.5% and 3.0% w/w) in hydrogels and TO concentrations (10.0%, 15.0% and 20.0% w/w) in organogels, with three replicates while maintaining constant concentrations of all other components. Only stability was used as the response parameter for analysis of the 27 bigel samples obtained.
Based on the results of the factorial design, a formulation improvement was proposed through a central composite experimental design with one central point and six replicates, varying HEC concentration (1.0% to 3.0% w/w) in hydrogels and oil concentration (2.0% to 6.0% w/w) in organogels.
Stability, pH and spreadability were used as response parameters for analysis of the 16 bigel samples prepared while maintaining constant concentrations of all other components. All experiments of the central composite experimental design were conducted with and without propylene glycol in the aqueous phase.
ANOVA and linear regression were performed with the response variables. All values were expressed as mean ± standard deviation (SD), and significance was considered at p-value < 0.05; statistical analysis was determined by softwareh.
Results: Tucuma Butter Bigel
Firstly, TB bigels were prepared using two different hydrogel gelators. TB was chosen to compose the oil phase to increase viscosity and, theoretically, the stability. CMC (anionic polymer) and HEC (nonionic polymer) were used to evaluate how the different charge and rheological behavior of the hydrogel interfered with bigel stability. The composition of TB bigels and the results of stability and spreadability are described in Table 1.
The stabilizer/emulsifier (4.00% w/w), methylparaben (0.10% w/w) and propylparaben (0.10% w/w) were constant in all formulations, and water was added in sufficient amount to make 100 g of the bigel (see Table 1). All 10 samples were slightly yellow and homogeneous.
After 24 hr, the prepared samples were centrifuged and visually inspected for phase separation. Samples N2, N3, N4, N5 and C2 were the only samples that remained homogeneous after centrifugation, while the others presented phase separation. The homogeneous samples were then submitted to spreadability evaluation, which is shown in Figure 1. Bigel sample N4, containing HEC 2% w/w and 10% w/w TB, showed a spreadability three times greater (3.51 mm²/g) than C2, composed of CMC 2.00% w/w and TB 15% w/w.
The bigel microstructures were observed by optical microscopy; micrographs are displayed in Figure 2. The sample N3 micrograph (left) clearly shows the internal phase is oily, confirming an organogel-in-hydrogel (o/h) bigel formation due to the presence of characteristic crystals of natural butters in the inner phase. The N4 micrograph (right) also shows an o/h bigel formulation with a homogeneous rounded inner phase dispersed in the outer phase. After 15 days at room temperature, all samples exhibited phase separation, which seemed to be due to TB crystallization, leading to bigel destabilization.
Tucuma Oil Factorial Model 3x3 DoE
TO was used to replace TB to confirm and overcome destabilization due to solid lipid crystallization. Twenty-seven randomized samples were prepared as described above; their compositions and analysis results are shown in Table 2. TO bigels were visually similar to TB bigels.
After 24 hr of preparation, the samples were centrifuged. Statistical analysis of the results showed that neither HEC nor TO concentration influenced bigel stability after centrifugation (p-value = 0.334 and p-value = 0.526, respectively). After 15 days at room temperature, all samples exhibited phase separation. Bigel instability seemed to relate to the high TO concentration of the tested formulations, which could increase interfacial tension, leading to repulsion between aqueous and oil phases.
Tucuma Oil Central Composite Experimental Design
TO central composite design experiments were then conducted to understand the interactions between components on bigel characteristics. The goal was to determine the optimal proportions to achieve an ideal bigel formulation with maximum spreadability.
All samples were prepared as described above; their compositions are summarized in Table 3, as well as pH, spreadability, water loss temperature and stability before and after the addition of PPG to the formulation. Table 4 displays the effects of model terms and associated p-values of the responses.
After 24 hr of preparation, the pH of the samples was measured and statistical analysis indicated that neither HEC nor TO proportions influenced bigel pH, as shown in Table 3. Although an increase in oil content was expected to raise pH due to the high fatty acid content of vegetable oils, it appears that the anionic polymer prevented the ionization of the carboxylic acid groups in the bigel.
Samples were also subjected to the spreadability test; the results are depicted in Figure 3. Statistical analysis revealed that HEC concentration significantly influenced bigel spreadability (p-value < 0.001), while TO concentration did not (p-value = 0.510; see Table 4).
Figure 4 illustrates the primary effects of HEC and TO concentration on the stability, pH and spreadability of bigel. The impact of HEC concentration on spreadability is negative, indicating that higher HEC concentrations correspond to lower spreadability, as depicted in Figure 4. Notably, for cosmetic formulations, achieving higher spreadability is desirable to enhance consumer compliance and experience.
The contour plot of spreadability and component proportions is depicted in Figure 5, also illustrating that an increase in HEC concentration results in a decrease in spreadability. This influence exhibits slight linearity (p-value < 0.001; R2 = 0.688) within the tested concentrations, reaching a plateau at around 2.4% HEC, as demonstrated in Figure 4.
Two thermal events were observed in the bigel thermograms. The first event corresponded to the evaporation of water at around 100ºC, while the second event occurred around 315ºC due to TO decomposition. Analysis presented in Table 3 indicates that the temperature of water loss event does not exhibit a significant tendency to increase with increasing TO concentration, and neither HEC nor TO ratios significantly influence the thermal stability of bigels (p-value = 0.819 and p-value = 0.649, respectively; see Table 4).
The centrifuging test aimed to assess the influence of HEC and TO concentration on bigel stability. Results indicated there was no significant effect of HEC or TO concentration on bigel stability when the formulation was prepared without propylene glycol (PPG), a humectant commonly used to minimize formulation water loss (p-value= 0.385 and p-value= 0.385, respectively; see Table 4). However, after 30 days of preparation, all bigel formulations exhibited phase separation, with water droplets observed in the flask used for storage at room temperature.
All samples of the central composite experimental design were then prepared with 5% (w/w) PPG on the HEC hydrogel, and after 24 hr, pH and centrifuging tests were conducted. The addition of the humectant did not cause a significant variation in pH, and neither HEC nor TO influenced the pH variation significantly (p-value= 0.060 and p-value= 0.289, respectively; see Table 4).
However, samples T4 and T5, both with lower concentrations of HEC, demonstrated phase separation after centrifuging when PPG was added to the bigel. The contour plot of the components' influence on stability is shown in Figure 6, illustrating the variation of stability according to the ratios of the components and highlighting the influence of both components on the response.
Statistical analysis reveals that HEC concentration positively affects bigel stability; as HEC concentration increases, bigel stability increases (p-value < 0.001; see Table 4). Conversely, the increase of TO leads to a decrease in stability (p-value = 0.015).
The effect of HEC concentration on bigel stability is greater than that of the TO concentration (+0.422 and -0.177, respectively), and the combined effect of both factors results in a positive effect on stability of 0.493 – larger than the effect of HEC alone. This suggests that the 3D network and mechanical properties of the external phase of the bigel influence its stability, with the internal phase concentration also playing a role.
Discussion
The results demonstrate that the choice of hydrogelator significantly influences the stability and spreadability of TB-based bigels. The inclusion of TB in the oil phase was intended to enhance viscosity and, theoretically, improve bigel stability. However, stability outcomes varied depending on the polymer used in the hydrogel phase.
While CMC (anionic) and HEC (nonionic) were employed to investigate how charge differences and rheological properties impact bigel behavior, only a subset of formulations exhibited stability after centrifugation. The phase separation of samples indicated that specific polymer concentrations and TB ratios contribute to the formation of a more robust gel network capable of withstanding mechanical stress.
Spreadability results further highlight the role of hydrogel composition in determining bigel performance. Notably, the sample with higher concentration of HEC and lower concentration of TB exhibited a spreadability greater than the samples with CMC and higher concentrations of TB. This suggests that the nonionic polymer (HEC) contributes to a less rigid but more deformable gel network, allowing for improved spreadability.
In contrast, the higher TB concentration may have led to increased rigidity, reducing the ability of the formulations to spread efficiently. This aligns with previous findings where polymer charge and molecular interactions modulate the rheological properties of gels, impacting their application characteristics.20, 21
Microscopic analysis provided further insight into the internal structure of the bigels. The presence of natural butter crystals in N3 and the rounded, dispersed oil phase in N4 confirm the formation of an organogel-in-hydrogel (o/h) system. This microstructural organization likely contributes to the observed differences in spreadability, as a well-dispersed oil phase can enhance the smoothness and application properties of the formulation.
Despite initial stability in some formulations, all samples exhibited phase separation after 15 days at room temperature. This suggests that TB crystallization is a critical factor contributing to long-term instability. The progressive crystallization of TB likely disrupts the gel network, leading to the collapse of the structured system and phase separation.
These findings indicate that further optimization is needed to prevent crystallization, possibly by adjusting TB concentration, incorporating stabilizing agents, modifying the cooling and storage conditions to control crystallization kinetics, or substituting TB with TO.22
The central composite design experiments provided valuable insights into the interactions between bigel components, particularly the effects of HEC and TO concentrations on physicochemical properties such as pH, spreadability, thermal stability and overall formulation stability. Statistical analysis revealed that HEC concentration significantly influenced bigel spreadability, whereas TO concentration had no measurable effect.
This suggests the polymeric network formed by HEC plays a dominant role in defining the mechanical properties of the formulation, corroborating findings by Rehman, et al.,9 who observed a reduction in bigel hardness with decreased hydrogel proportion in hydroxypropylmethylcellulose (HPMC) bigels.
Similarly, Shakeel, et al.,7 emphasized that hydrogel content is crucial in determining bigel firmness and strength due to hydrogen bonding, particularly in o/h systems.7
Spreadability tests confirmed that higher HEC concentrations resulted in lower spreadability, a trend consistent with previous studies showing that increased hydrogel content leads to firmer, less deformable structures. This observation aligns with findings by Singh, et al.,8 who reported that lower organogel concentrations led to increased spreadability in bigels containing carbomer and sorbitan monostearate with sesame oil.
However, the current results also indicate that TO concentration does not significantly influence spreadability, which contrasts with expectations from traditional oil-in-water emulsions, where viscosity typically increases with higher oil content in the dispersed phase. Lupi, et al.,5 similarly noted that increasing oil content in bigel formulations does not necessarily result in increased viscosity, further supporting the idea that mechanical properties are predominantly governed by the external phase rather than the dispersed phase in o/h bigels.
Thermal analysis revealed two distinct events: water loss around 100°C and TO decomposition at approximately 315°C. However, no significant relationship was found between TO concentration and the temperature of the water loss event, nor did HEC or TO significantly impact the thermal stability of the bigel. This contrasts with the findings of Rheman, et al.,9 who reported an increase in thermal stability with higher fish oil content in HPMC-based bigels.
This discrepancy suggests that the influence of oil content on thermal stability may be formulation-dependent, with differences in polymer type, oil phase composition and the overall structural arrangement playing key roles.
The stability evaluation through centrifugation tests demonstrated that neither HEC nor TO concentrations had a significant effect on bigel stability in the absence of PPG. However, after 30 days, all formulations exhibited phase separation, likely due to water evaporation and interfacial forces between the aqueous and oil phases. To address this issue, PPG was incorporated into the hydrogel phase to enhance water retention and improve stability. Following the addition of PPG, statistical analysis revealed that HEC concentration had a significant positive effect on bigel stability, whereas TO exhibited a negative influence.
The combined effect of HEC and TO yielded a greater positive impact on stability than the individual effect of HEC alone, suggesting that the structural integrity of the hydrogel matrix plays a pivotal role in stabilizing the system. These findings contrast with previous studies, such as by Singh, et al.,8 who observed that an increase in sesame oil content led to enhanced stability in h/o systems containing guar gum.
The current study results suggest that the stability of o/h bigels may be more dependent on the external phase composition rather than the oil content itself. The role of the hydrogel matrix is further reinforced by the observation that, after PPG addition, samples with lower HEC concentrations exhibited phase separation after centrifugation. This finding aligns with prior studies emphasizing that hydrogen bonding and polymer network interactions are crucial for maintaining bigel stability.7
Conclusions
In conclusion, these results indicate that HEC concentration is the key determinant of bigel spreadability and stability, with TO exerting a secondary influence. Unlike traditional emulsions, where increased oil content often enhances viscosity and stability, the behavior of bigels appears to be more complex, governed primarily by the external hydrogel matrix.
The use of Design of Experiments (DoE) proved to be a valuable tool in understanding these interactions, as it allowed for the systematic evaluation of multiple factors simultaneously, highlighting the significant influence of HEC and TO concentrations on key formulation attributes.
The central composite design experiments enabled the identification of the optimal component proportions to achieve a stable bigel with desirable spreadability. Through statistical analysis, DoE provided insights into the relationship between formulation variables and performance metrics, such as pH, stability and rheological properties.
The ability to predict and optimize these characteristics through DoE is particularly relevant for the development of cosmetic and pharmaceutical bigels, where achieving both stability and appropriate mechanical properties is crucial for product efficacy and consumer acceptance.
Moreover, the DoE approach facilitated the identification of complex interactions between the hydrogel and oil phases, demonstrating that HEC concentration plays a dominant role in determining the mechanical behavior of the bigel. This systematic approach also helped in understanding the limitations of the formulation, such as the impact of TB crystallization on long-term stability and the role of PPG in improving water retention.
Future studies should focus on further refining DoE models to explore additional formulation parameters, such as different polymer types, oil phase modifications and alternative stabilizing agents. By leveraging DoE methodologies, researchers can continue to optimize bigel formulations to achieve enhanced stability, spreadability and overall performance, ensuring their suitability for various pharmaceutical and cosmetic applications.
Acknowledgements: This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Footnotes
a Engenharia das Essências, São Paulo, Brazil
b Amazon Oil, Pará, Brazil
c Emulfree P (INCI: Ethylcellulose (and) Propylene Glycol Isostearate (and) Propylene Glycol Laurate) is a product of Gatefossé, Paris, France.
d UltraStirrer D500, Scilogex, USA
e Olympus BX51, Japan
f TA Q500, USA
g PHS-3E, China
h Minitab17 software
References
- Dickinson, E. (2003). Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocolloids, 17(1) 25–39.
- Singh, V.K., Anis, A., Banerjee, I., Pramanik, K., Bhattacharya, M.K. and Pal, K. (2014). Preparation and characterization of novel carbopol based bigels for topical delivery of metronidazole for the treatment of bacterial vaginosis. Materials Science and Engineering: C, 44 151–158.
- Ibrahim, M.M., Hafez, S.A. and Mahdy, M.M. (2013). Organogels, hydrogels and bigels as transdermal delivery systems for diltiazem hydrochloride. Asian Journal of Pharmaceutical Sciences, 8(1) 48–57.
- Esposito, C.L., Kirilov, P. and Roullin, V.G. (2018). Organogels, promising drug delivery systems: An update of state-of-the-art and recent applications. Journal of Controlled Release, 271 1–20.
- Lupi, F.R., Gentile, L., Gabriele, D., Mazzulla, S., Baldino, N. and de Cindio, B. (2015). Olive oil and hyperthermal water bigels for cosmetic uses. Journal of Colloid and Interface Science, 459 70–78.
- Rehman, K. and Zulfakar, M.H. (2017). Novel fish oil-based bigel system for controlled drug delivery and its influence on immunomodulatory activity of imiquimod against skin cancer. Pharmaceutical Research, 34(1) 36–48.
- Shakeel, A., Lupi, F. R., Gabriele, D., Baldino, N., & Cindio, B. D. (2018). Bigels: A unique class of materials for drug delivery applications. Soft Materials, 16(2), 77–93.
- Singh, V. K., Banerjee, I., Agarwal, T., Pramanik, K., Bhattacharya, M. K., & Pal, K. (2014). Guar gum and sesame oil-based novel bigels for controlled drug delivery. Colloids and Surfaces B: Biointerfaces, 123, 582–592.
- Rehman, K., Amin, M.C.I.M. and Zulfakar, M.H. (2014). Development and physical characterization of polymer-fish oil bigel (hydrogel/oleogel) system as a transdermal drug delivery vehicle. Journal of Oleo Science, 63(10) 961-970.
- Almeida, I. F., Fernandes, A.R., Fernandes, L., Ferreira, M.R.P., Costa, P.C. and Bahia, M.F. (2008). Moisturizing effect of oleogel/hydrogel mixtures. Pharmaceutical Development and Technology, 13(6) 487–494.
- Tian, W., Huang, Y., Song, Z., Abdullah, Yu, Y., Liu, J., Cao, Y. and Xiao, J. (2023). Flexible control of bigel microstructure for enhanced stability and flavor release during oral consumption. Food Research International, 174, 113606.
- Tian, W., Huang, Y., Liu, L., Yu, Y., Cao, Y. and Xiao, J. (2024). Tailoring the oral sensation and digestive behavior of konjac glucomannan-gelatin binary hydrogel based bigel: Effects of composition and ratio. International Journal of Biological Macromolecules, 256 127963.
- Kouider Amar, M., Rahal, S., Laidi, M., Kouar, I., Bourahla, R.F.E.-K., Akouche, Y. and Bouaraba, R. (2024). Balancing competing objectives in bigel formulations using many-objective optimization algorithms and different decision-making methods. European Journal of Pharmaceutics and Biopharmaceutics, 195 114167.
- Baldissera, M.D., Souza, C.F., ... Monteiro, S.G., et al. (2017). Antihyperglycemic, antioxidant activities of tucumã oil (Astrocaryum vulgare) in alloxan-induced diabetic mice, and identification of fatty acid profile by gas chromatograph: New natural source to treat hyperglycemia. Chemico-Biological Interactions, 270 51–58.
- Nascimento, K., Copetti, P.M., ... da Silva, J.E.P., et al. (2021). Phytochemical analysis and evaluation of the antioxidant and antiproliferative effects of Tucumã oil nanocapsules in breast adenocarcinoma cells (MCF-7). Natural Product Research, 35(12) 2060–2065.
- Oboh, F.O.J. and Oderinde, R.A. (1988). Analysis of the pulp and pulp oil of the tucum (Astrocaryum vulgare Mart) fruit. Food Chemistry, 30(4) 277–287.
- Kahn, F. (2008). The genus Astrocaryum (Arecaceae). Revista Peruana de Biología, 15 31–48.
- Rossato, A., Silveira, L. da S., ... Sagrillo, M.R., et al. (2019). Evaluation in vitro of antimicrobial activity of tucumã oil (Astrocaryum vulgare). Archives in Biosciences and Health, 1(1) Artigo 1.
- Shakeel, A., Farooq, U., Iqbal, T., Yasin, S., Lupi, F.R. and Gabriele, D. (2019). Key characteristics and modelling of bigels systems: A review. Materials Science and Engineering: C, 97 932–953.
- Hou, J., Liu, Y., ... Qin, D., et al. (2025). Charge density of carboxymethyl cellulose affects depletion attraction-stabilized egg yolk Pickering emulsion gels: Rheological and interfacial properties. Food Hydrocolloids, 159 110612.
- Talló, K., Vílchez, S., Pons, R. and López, O. (2021). Gels formed from the interaction of lipid vesicles: Influence of charge in their structural and rheological properties. Journal of Molecular Liquids, 322 114957.
- Li, L. and Liu, G. (2023). Engineering effect of oleogels with different structuring mechanisms on the crystallization behavior of cocoa butter. Food Chemistry, 422 136292.
- Roy, H., Maddela, S., Munagala, A., Rahaman, S.A. and Nandi, S. (2021). A quality by design approach of metronidazole bigel and assessment of antimicrobial study utilizing Box-behnken design. Combinatorial Chemistry & High Throughput Screening, 24(10) 1628–1643.










