The aging process leads to a loss of firmness and elasticity in the skin, causing tissues to collapse and the subsequent production of wrinkles. This wrinkle formation involves dramatic structural alterations in the epidermis, dermis and hypodermis. For example, alterations of the hydration state and water-holding capacity of the epidermis have been found to contribute significantly to the formation of wrinkles1 and can easily be associated with a deficiency of water mostly due to the drying activity of environmental factors such as UV, light, heat and pollution.2
The epidermis is mainly composed of keratinocytes, which proliferate in the stratum basale (SB), differentiate and migrate toward the skin surface. The outermost layer of the epidermis, the stratum corneum (SC), is mostly composed of flattened and cornified keratinocytes, which produce high amounts of keratin during the differentiation process. Dead keratinocytes are held together by junctions and microfibrils composed of different insoluble proteins that are essential for the skin’s mechanical integrity and waterproof properties.
Involucrin (INV) and filaggrin (FLG) are two important proteins in the epidermis, and both contribute to the structural integrity of the SC.3 Moreover, FLG is a source of natural moisturizing factors (NMF), as it generates precursors of the NMF upon hydrolysis.4 Another important factor that plays a key role in skin hydration and integrity of the epidermis is aquaporin (AQP); specifically, aquaporin 3 (AQP3), located at the plasma membrane in the SB and stratum spinosum, where it plays an essential role in water transport.5
In the dermis, the fibroblasts are mainly responsible for the production of all the components of the extra-cellular matrix (ECM), such as collagen, elastin, hyaluronan and proteoglycans. The formation of wrinkles is associated with changes in the elastic and supporting proprieties of the ECM, mainly due to the denaturation of the collagen fibers, and/or to an unbalanced ratio of collagen synthesis and degradation.6 Visible signs of aging also appear as the adipocytes present in the hypodermis cannot fill in the skin and compensate for damages in the outer layers. Adipocytes provide volume and help add thickness and suppleness to the skin.7 In time, there is a decline in the capacity of pre-adipocytes to differentiate and of fat cells to create new lipids. This contributes to a decrease in fat mass and to alterations in fat tissue function.8
Coffea Extract
Extracts obtained from Coffea plants have long been recognized for their health-enhancing potential, including antioxidant capacity9 and immunomodulation.10 Due to high levels of antioxidant compounds present in such extracts, which are known to protect the skin from free radicals and prevent the oxidation of proteins such as collagen, it was hypothesized that the extracts may also exhibit antiwrinkle activity. However, although caffeine has been associated with anticancer and UV protection activity, it also has been shown to reduce the thickness of the hypodermis,11 which favors wrinkle formation. Therefore, with the purpose of developing a plant cell extract that exhibits the beneficial properties of Coffea but also inhibits wrinkle formation, an extract from liquid cultured cells of Coffea bengalensis was obtained since this species does not contain caffeine. The extract was then tested for its capacity to enhance skin hydration in the epidermis, to stimulate collagen synthesis in the dermis and to induce adipocyte differentiation in the subcutaneous layer, as is described later in this article.
Plant stem cell cultures are useful in obtaining extracts with specific and desired characteristics for cosmetics.12, 13 Their totipotency and ability to behave as differentiated cells under specific conditions, allows plant stem cells to represent a more versatile biofactory system than whole plants. Moreover, extracts obtained from stem cell cultures, which are grown under controlled laboratory conditions and in the absence of contaminants and pathogens, are completely standardized. Thus, the characteristics of quality of the product are guaranteed and do not depend upon the time and season of collection, as for the plants.
Materials and Methods
To obtain the active from Coffea cells, preparations were made from liquid cultures of C. bengalensis stem cells obtained by the proliferation of plant calluses. Leaves of C. bengalensis plants were surface sterilized in 70% ethanol for 15 min, 1% bleach for 15 min and washed three times with sterile water. The leaf explants were cut in 0.5 cm pieces, wounded with a scalpel blade and put for callus induction on Gamborg B5 medium, supplemented with myo-inositol (500 mg/L), sucrose (30 g/L), 2.4 D (1 mg/L), kinetin (0.1 mg/L), adenine (1mg/L) and plant agar (7.5mg/L). White and friable calli were obtained from the proliferation of the epidermis cells of the leaf after five weeks of cultivation in the dark. The callus cultures were maintained by transferring the tissues on fresh medium every four weeks. To initiate stem cell liquid culture, 50 mg of 40–45 day old callus was resuspended in 30 mL of liquid medium. The culture was incubated at 27°C in the dark under constant orbital stirring (110 rpm).
The callus tissue gradually disintegrated and formed single cells or small cell aggregates after 10 days. Then the suspensions were transferred into bigger volumes, where 50 mL of a dense culture was used as starting material for inoculating 1 L of culture medium. To prepare the extracts, the cells were collected and homogenized, and the resulting lysate was centrifuged at 10,000 rpm to precipitate the particulate fraction and isolate the soluble fraction, which was collected and lyophilized. The powder obtained was then dissolved in water at the required concentrations, described later.
As previously mentioned, it is known from the literature that the species C. bengalensis, different from C. arabica, contains low levels of caffeine.14 Thus, the cultured stem cells obtained from de-differentiation of leaf cells were expected to contain undetectable levels of the alkaloid. This hypothesis was confirmed by a high-performance liquid chromatography analysis, which revealed the absence of caffeine and any other alkaloid in the extract samples tested. To demonstrate the antiwrinkle effect of the Coffea cell extract, a series of experiments was then conducted using different skin cell lines, each corresponding to a specific skin layer.
AQP3, FLG and INV gene expression analysis: Keratinocytes (HaCaT) were pre-incubated for 6 hr with the test compounds then collected for total RNA extraction. Total RNA was extracted using an RNA purification kita according to the manufacturer’s instructions. Reverse transcription polymerase chain reaction (RT-PCR) was performed using gene-specific primers and an oligo-nucleotide internal standard kit,b also according to the manufacturer’s instructions. The PCR products obtained were visualized and quantified with an imaging systemc. The amplification band corresponding to the gene analyzed was normalized to that corresponding to the kit. The values obtained were converted into percentages by considering the result of the untreated controls as 100%.
ELISA collagen synthesis analysis: Newly synthesized collagen was measured by enzyme-linked immunosorbent assay (ELISA). NIH3T3 fibroblasts were seeded in 96-well plates and treated with test compounds for 24 hr. Cells were then fixed in formaldehyde (4%) and incubated with primary goat monoclonal antibodies raised against pro-collagen type I and type IIId. Samples were incubated 2 hr later with horse radish peroxidase (HRP)-labeled anti-goat secondary antibody. The amount of new collagen produced by the cells was measured by a colorimetric reaction using the reagent o-phenylenediamine (OPD) as the substrate.
Adipocyte differentiation: Human mesenchymal stem cells (MSC) were plated in 96-well plates and grown at 37°C to 100% confluence. Two-day, post-confluent cells were incubated in a specific adipogenesis-inducing medium (AIM) for three days, then incubated for three additional days in adipogenesis maintenance medium (AMM) and finally switched to AIM again. The switch from AIM to AMM and back to AIM was repeated for three cycles. The test compounds were added to the medium for the whole differentiation period. On day 21, intracellular lipid content was measured using a specific fluorescent dye for lipidse.
ATP assay: NIH3T3 fibroblasts were seeded in 96-well plates and grown for 20 hr. The cells were then incubated with different concentrations of test compounds for 2.5 hr. To measure the adenosine triphosphate (ATP) content in the cells, an equal volume of detection reagentf was added to each well, and the luminescent signal of the samples was measured by using a plate readerg.
Reactive oxygen species (ROS) assay: NIH3T3 fibroblasts, also grown in 96-well plates for 20 hr, were incubated with different concentrations of test compound or ascorbate 250 μM (positive control) for 2 hr. The cells were then incubated with the dye (5-(6-)chloromethyl-2,7-dichloro-dihydrofluorescein diacetateh at 37°C for 30 min. After treatment with H2O2 150 μM, the fluorescence of the samples was measured at 535 nm using a dual detector instrumentj.
Results and Discussion
Keratinocytes treated with C. bengalensis cell extract in sterile distilled water were shown to increase the expression of genes involved in skin hydration and protection against water loss, compared with control samples, indicated as untreated or control in the figures. As shown in Figure 1, at a concentration of 0.05%, the extract increased the expression of INV and FLG by 40% and 42%, respectively. The extract also augmented the expression of AQP3 by 120%, suggesting a specific role in the maintenance of water balance to correct the hydration of the upper skin layers.
Concerning the dermis, C. bengalensis cell extract was able to induce collagen synthesis in cultured fibroblasts. After treatment with the extract at a concentration of 0.03%, the production of new collagen (pro-collagen) of type I and III increased by 80% and 45%, respectively, suggesting it could also have a firming activity in the dermis (see Figure 2).
As previously described, the hypodermis contains adipocytes, which differentiate from precursor cells called mesenchymal stem cells (MSC). These cells have the capacity for self-renewal and produce new adipocytes that are responsible for “replumping” the skin.15 MSC were treated with C. bengalensis cell extract and nicotinamide (positive control) for its capacity to stimulate adipocyte differentiation.16 Then, the differentiated adipocytes were detected using a specific dye. The values obtained illustrated that the cell extract had a strong stimulatory effect on adipocyte differentiation, indicating its potential capacity to counteract the collapse of tissues caused by aging (see Figure 3).
The in vitro results thus far suggest that C. bengalensis cell extract acts independently on each of the three main layers forming the skin, activating mechanisms of hydration, firming and replumping, which are all important to reducing the visible signs of aging (see Figure 4). Besides its beneficial antiwrinkle effects, C. bengalensis cell extract also reduced intracellular ROS by up to 60%, indicating it is capable of protecting the cells from free radical damage. Moreover, it produced a 35% increase of ATP in the cells, as shown in Figure 5.
While in vivo tests are under way, results are currently unavailable, although experience with similar data from testing similar cultured stem cell actives of other plant species has been confirmed by in vivo tests. Further, cell extracts contain many different compounds, from small antioxidant molecules to bigger peptides and proteins, and according to other studies, compounds with a molecular weight lower that 500 d can easily pass through the epidermis and diffuse through the dermis and hypodermis layer.17 Moreover, the capacity of the compounds to reach the lower skin layers mostly depends on the degree of lipophilicity of these compounds, and most of the antioxidant compounds present in Coffea extracts such as gallic acid, caffeic acid, sinapic acid and diterpenes, together with amino acids derived from protein hydrolysis, are small and possess hydrophobic chemical groups. Thus, they are able to diffuse across the upper skin layers of the skin.18
Formulating With C. bengalensis
Two examples of formulations that include the C. bengalensis stem cell extract at 0.5% are shown in Formulas 1 and 2. The Coffea cell extract is first diluted in water, then in glycerol for final use in cosmetic formulas. The blend produced is transparent with a light coloration—i.e., yellow to brownish, odorless and preservative free. It is fully soluble in aqueous medium, in glycerin and ethanol but due to its hydrophilic nature, it can be hardly incorporated in anhydrous systems. Overall, however, it is compatible with a variety of cosmetic preparations.
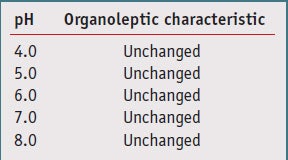
The extract also has been shown to remain stable at pH ranges from 4.0 to 8.0, as illustrated in Table 1. Additionally, it can be added to cosmetic preparations either during the cooling phase or at the end of the formulation, as long as the temperature is below 70°C. Furthermore, the antioxidant activity of the Coffea cell extract remains unchanged after being dissolved in medium such as glycerol, as measured by oxygen radical absorbance capacity (ORAC) assay.
Conclusions
According to the in vitro and in cell assays described, which are typically predictive of the efficacy of extracts in vivo, C. bengalensis stem cell extract may find interesting applications as a cosmetic active. This extract is based on a versatile plant cultured stem cell technology in which the most appropriate plant species and cultivars—i.e., those containing compounds with the strongest specific activity in the desired field—are chosen and developed to produce standardized actives. Further, the process for developing the extract addresses various environmental aspects such as the preservation of biodiversity, and safety and ecological sustainability, which are priorities for today’s market.
References
Send e-mail to [email protected].
1. JL Contet-Audonneau, C Jeanmaire and G Pauly, A histological study of human wrinkle structures: Comparison between sun-exposed areas of the face, with or without wrinkles, and sun-protected areas, Brit J Dermatol 140, 1038–1047 (1999)
2. T Sano, T Kume, T Fujimura, H Kawada, S Moriwaki and Y Takema, The formation of wrinkles caused by transition of keratin intermediate filaments after repetitive UVB exposure, Arch Dermatol Res 296, 359–365 (2005)
3. T Mammone, M Ingrassia and E Goyarts, Osmotic stress induces terminal differentiation in cultured normal human epidermal keratinocytes, In Vitro Cell Dev Biol 44, 135–139 (2008)
4. RA van den Oord and A Sheikh, Filaggrin gene defects and risk of developing allergic sensitization and allergic disorders: Systematic review and meta-analysis, BMJ 339, b2433 (2009) 5. M Hara-Chikuma and AS Verkman, Roles of aquaporin-3 in the epidermis, J Invest Dermatol 128, 2145–2158 (2008)
6. C Flynn and BAO McCormack, Simulating the wrinkling and aging of skin a multi-layer finite element model, J Biomech 43, 442–448 (2010)
7. P Quatresooz, L Thirion, C Pierard-Franchimont and GE Pierard, The riddle of genuine skin microrelief and wrinkles, Intl J Cosm Science 28, 389–395 (2006)
8. M Berneburg, Research in practice: More that skin deep-aging of subcutaneous fat tissue, J Germ Soc Dermatol 10, 776–778 (2010)
9. M Serafini and MF Testa, Redox ingredients for oxidative stress prevention: The unexplored potentiality of coffee, Clin Dermatol 27, 225–229 (2009)
10. T Kobayashi, M Yasuda, K Lijima, K Toriizuka, JC Cyong and H Nagasawa, Effects of coffee cherry on the immune system in SHN mice, Anticancer Res 16, 1827–1830 (1997)
11. Y Lou, Q Peng, B Nolan, GC Wagner and Y Lu, Oral administration of caffeine during voluntary exercise markedly decreases tissue fat and stimulates apoptosis and cyclin B1 in UVB-treated skin of hairless p53-knockout mice, Carcinogenesis 31 (4) 671–678 (2010)
12. A Barbulov et al, Raspberry stem cell extract to protect skin from inflammation and oxidative stress, Cosm &Toil 125 38–47 (2010)
13. A Tito et al, A tomato stem cell extract, containing antioxidant compounds and metal chelating factors, protects skin cells from heavy metal-induced damages, Int J Cosmet Sci May 24 (2011)
14. H Ashihara and A Crozier, Biosynthesis and catabolism of caffeine in low caffeine-containing species of Coffea, J Agric Food Chem 47, 3425–3431 (1999)
15. M Wu, L Yang, S Liu, N Hui, F Wang and H Liu, Differentiation potential of human embryonic mesenchymal stem cells for skin-related tissue, Cut Biol 155, 282–291 (2006)
16. CM Backesjo, Y Li, U Lindgren and LA Haldosen, Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells, J Bone Miner Res 21 (7), 993–1002 (2006)
17. JD Bos and MMHM Meinardi, The 500 Dalton rule for the skin penetration of chemical compounds and drugs Exp Dermatol 9 165–169 (2000)
18. A Farah, M Monteiro, CM Donangelo and S Lafay, Chlorogenic acids from green coffee extract are highly bioavailable in humans, J Nutr 138 2309–3215 (2008)