Fragrances are complex combinations of substances that are added to many consumer products to give them a distinctive smell, impart a pleasant odor, or mask the inherent smell of some ingredients—but ultimately, to enhance the experience of the product user. Fragrances create important olfactory benefits that are ubiquitous, tangible and valued. They can be used to communicate complex ideas such as creating a mood; signaling cleanliness, freshness or softness; alleviating stress; providing a sense of well-being; or to trigger allure and attraction. In most types of cosmetics and skin care products, including perfumes, shampoos, conditioners, moisturizers, facial cosmetics and deodorants, there are more than 5,000 different fragrances present.
In relation, many people suffer from allergies, which can be introduced in a number of ways including inhalation, ingestion, injection or skin contact. Allergies are often manifested as itchy eyes, a runny nose, wheezing, skin rashes (including dermatitis1) or diarrhea. In the European Union (EU), Cosmetic Regulation 1223/2009 currently lists 26 fragrance ingredients, 24 volatile chemicals and two natural extracts (oak moss and tree moss) that are considered more likely to cause reactions in susceptible people.2 These 26 ingredients must be indicated on the final product ingredient disclosure if the concentration exceeds 0.001% (10 mg/kg) in leave-on products, e.g. moisturizers, or 0.01% (100 mg/kg) in rinse-off products, e.g., shampoos.
Listing the 26 regulated allergens on products not only helps consumers to identify the cause of an allergic reaction, it also aids in their choosing what product to buy initially, particularly if they have a diagnosed allergy to a specific fragrance ingredient. Current analytical methods used for the analysis of cosmetic allergens include: gas chromatography mass spectrometry3-5 (GC-MS), headspace GC-MS,6 GC-GC/MS, liquid chromatography-UV (LC-UV)7 and LC-MS,8 all of which have run times of approximately 30 to 40 min.
The 24 regulated volatile cosmetic allergens contain compounds from different classes and different polarities; i.e., phenols, cyclic hydrocarbons, alcohols, carbonyl compounds, esters and lactones. Many are small molecules with similar structures that often produce non-specific fragment ions for mass spectrometric detection. With these materials, there are many challenges to address for proper allergen analysis. For example, the resolutions achieved between analyte, isomer and matrix components all must be optimized, and the sensitivity of the method should be at least 1 ppm—although greater is preferred.
Convergence chromatography (CC) is a separation technique that uses carbon dioxide as the primary mobile phase with the option (if required) to use an additional co-solvent such as acetonitrile or methanol to give selectivity similar to normal phase LC. This method has been further developed, hyphenating Waters® UltraPerformance Convergence Chromatography™ (UPC2®) with MS detection for the analysis of fragrance allergens in perfume, cosmetics and personal care products.
Benefits of the method include:
- The ability to run LC- and GC-amenable compounds in a single analysis;
- Fast, 7-min analysis of the 24 regulated cosmetic allergens, 4 non-regulated cosmetic allergens, and 2 potential carcinogenic compounds containing:
- different classes of compounds and
- different polarities;
- An orthogonal technique that enables greater selectivity and specificity compared to either HPLC or GC analysis alone; and
- Besides the 7-minute method being more than six times faster than existing HPLC and GC methods, it uses 95% less solvent than existing HPLC methods.
For more information on this method, please download the complete application note. To view a wide range of application notes describing testing methods for raw materials, formulation, safety/regulatory, quality control and counterfeit detection, download Waters Cosmetics and Personal Care Application Book .
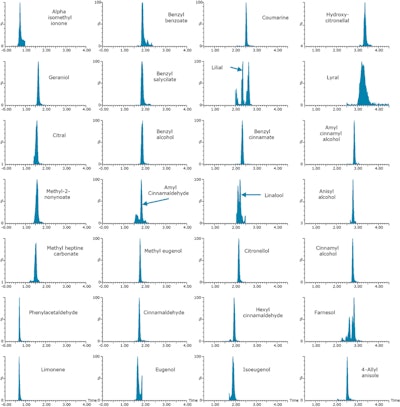
Figure 1. Perfume samples were fortified at 10 mg/kg (0.001%) with 24 cosmetic allergens and four additional compounds. Samples were analyzed with the ACQUITY UPC2 System and Xevo TQD tandem quadrupole mass spectrometer. Example MRM chromatograms achieved for fortified perfume are shown.
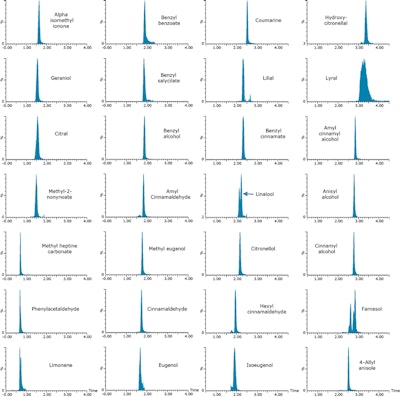
Figure 2. Shampoo samples were fortified at 100 mg/kg (0.01%) with 24 cosmetic allergens and 4 additional compounds. Samples were analyzed with the ACQUITY UPC2 System and Xevo TQD tandem quadrupole mass spectrometer. Example MRM chromatograms achieved for fortified shampoo are shown.
References
- Larson W, Nakayama H, Fischer T, et al. Fragrance contact dermatitis: a worldwide multicenter investigation (Part II). Contact Dermatitis. 44(6): 344–346, June 2001.
- The European Parliament and the Council of the European Union. Regulations (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Official Journal of the European Union. L 342/59: 59-209, 22nd Dec 2009. [cited 2015 January 15]. Available from: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:342:0059:0209:en:PDF
- Debonneville C, Chaintreau A. Quantitation of suspected allergens in fragrances: evaluation of comprehensive GCconventional MS. J Chromatogr A. 1027: 109–15, 2004.
- Rastogi S C. Analysis of Fragrances in Cosmetics by Gas Chromatography-Mass Spectrometry. J High Resolution Chrom. 18: 653–658, 1995.
- Lopez-Nogueroles M, Chisvert A, Salvador A. Determination of atranol and chloratranol in perfumes using simultaneous derivatization and dispersive liquid-liquid microextraction followed by gas chromatography-mass spectrometry. Analytica Chimica Acta. 826: 28–34, 2014.
- Desmedt B, Canfyn M, Pype M, et al. HS-GC-MS method for the analysis of fragrance allergens in complex cosmetic matrices. Talanta. 131: 444–451, 2015.
- Villa C, Gambaro R, Mariani E, Dorato S. High-performance liquid chromatographic method for the simultaneous determination of 24 fragrance allergens to study scented products. J Pharmaceutical and Biomedical Analysis. 44: 755–762, 2007.
- Rudback J, Islam N, Nilsson U, et al. A sensitive method for determination of allergenic fragrance terpene hydroperoxides using liquid chromatography coupled with tandem mass spectrometry. J Sep Sci. 36:1370–1378, 2013.
Waters. ACQUITY, UPC2, Xevo, and The Science of What's Possible are registered trademarks of Waters Corporation. Ultra Performance Convergence Chromatography is a trademark of Waters Corporation.
Disclaimer:
The above paid-for content was produced by and posted on behalf of the Sponsor. Content provided is generated solely by the Sponsor or its affiliates, and it is the Sponsor’s responsibility for the accuracy, completeness and validity of all information included. Cosmetics & Toiletries takes steps to ensure that you will not confuse sponsored content with content produced by Cosmetics & Toiletries and governed by its editorial policy.







!['[Sunscreen] developers will be able to innovate more efficiently while maintaining high standards of quality and safety for consumers.'](https://img.cosmeticsandtoiletries.com/files/base/allured/all/image/2024/06/woman_outside_using_sunscreen_on_face_ISO_test_standards_AdobeStock_783608310.66678a92029d9.png?auto=format%2Ccompress&fit=crop&h=191&q=70&rect=62%2C0%2C2135%2C1200&w=340)