
What’s the difference between something that is soluble vs. insoluble? In his monthly “Comparatively Speaking” column, Tony O’Lenick explores this notion. At first, he writes, this may seem quite simple but a deeper investigation provides formulation insight.
On a fundamental level, something that is soluble simply means it “goes into a solvent”; or solubility is “the ability of a substance to dissolve; the quality of being soluble.”1 Thus, we could correctly say that salt is water soluble and olive oil is water insoluble. While seemingly simplistic, it misses the point of understanding solubility as it pertains to the ability to formulate.
Continuing this definition, solubility could be defined as “The upper limit of solute that can be dissolved in a given amount of solvent at equilibrium.”1 Clearly, we are becoming more complex in our definition. In fact, the next step defines the situation even more fully.
“Solubility is a measure of the ability for a particular substance in a particular solvent, equal to the quantity of substance dissolving in a fixed quantity of solvent, to form a saturated solution under specified temperature and pressure. It is expressed in grams per cubic decametre, grams per hundred grams of solvent, moles per mole, etc.”1 It is interesting to note that these definitions all come from the same reference.
Defining Parameters
To explore solubility, several parameters must be defined in a given scenario. For the sake of simplicity, consider the present scenario as salt in water. First, we must consider:
1. The Compound
In this case of salt, which one? Sodium chloride, potassium chloride, sodium acetate or even sodium benzoate? As will become clear, identifying the compound is crucial. For the sake of this discussion, assume it is NaCl.
2. The Solvent
The solvent used also must be specified. Table 1 shows the effect of the solvent on the solubility of NaCl:2
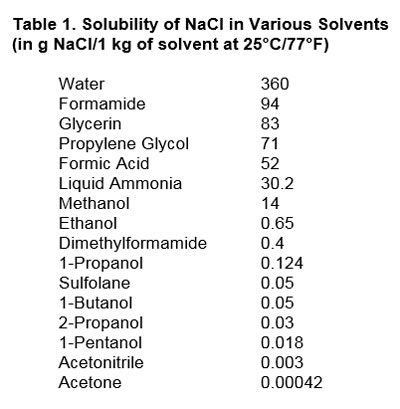 As can be seen, solubility varies quite a bit depending upon which solvent is used. The best solvent for NaCl is water, with a reported 36% by weight. The worst is acetone, with just 0.0042% by weight.
As can be seen, solubility varies quite a bit depending upon which solvent is used. The best solvent for NaCl is water, with a reported 36% by weight. The worst is acetone, with just 0.0042% by weight.Two points must be made here. The first is that NaCl is neither completely soluble in water—i.e., if one exceeds 36% by weight—nor is it completely insoluble in acetone, if one stays below the 0.0042% threshold. Second, solubility is temperature-dependent as well; thus:
3. The Temperature
Figure 1 shows the effects of temperature on solubility; reprinted from Ref 4:4, 5
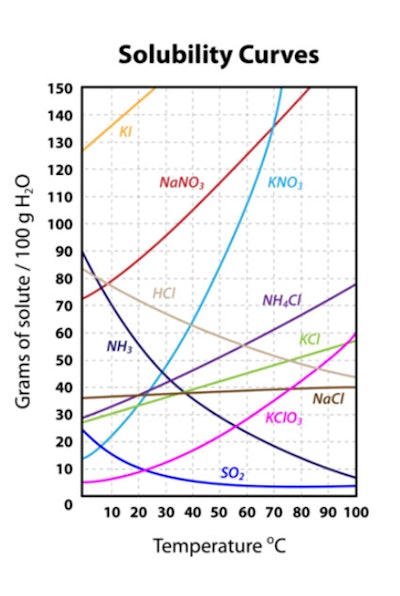
Clearly, temperature is a potential concern to formulations, as they may be stored in cold or hot environments; this can even apply to the body temperature of consumers using products.
Therefore, in a two-component system of salt in water, the compound, solvent and/or temperature can change the solubility and affect the saturation concentration. However, the personal care world is far more complex; rarely do systems comprise just two components. In general, most products include water, oil, several salts, preservative, fragrance and many other ingredients. These all interact with each other and alter solubility.
Partition Coefficient
When oil and water are present in the same formulation, a property known as the partition coefficient can help to understand where the compound(s) of interest will end up, and in what concentration. The addition of low concentrations of raw materials are known to partition into both aqueous and oil phases; an example is preservatives. In addition, it is well-understood that a small quantity of water added to an oil phase before emulsification will result in a smaller particle-sized, more stable emulsion; this is because water is not insoluble in oils.
The partition coefficient (P) or distribution coefficient (D) is the ratio of concentrations of a compound in a mixture of two immiscible phases at equilibrium. This ratio is therefore a measure of the difference in solubility of the compound in these two phases. The partition coefficient generally refers to the concentration ratio of un-ionized species of compound, whereas the distribution coefficient refers to the concentration ratio of all species of the compound—i.e., ionized plus un-ionized.5
In chemical and pharmaceutical sciences, both phases usually are solvents.6 One of the solvents is most commonly water while the second is typically something hydrophobic, such as 1-octanol.7 Hence, the partition coefficient is a measure of the level of hydrophilic ("water-loving") or hydrophobic ("water-fearing") nature in a given chemical substance.
Anyone who has performed an extraction in an organic chemistry lab has taken advantage of this partition coefficient, as shown by the separatory funnel in Figure 2, with the oil phase on top and water phase on the bottom:
 As stated, the partition coefficient is the ratio of concentrations of un-ionized compound between the two solutions. To measure the partition coefficient of ionizable solutes, the pH of the aqueous phase is adjusted such that the predominant form of the compound is un-ionized. The logarithm of the ratio of the concentrations of un-ionized solute in the solvents is called log P and calculated as follows (see Equation 1):10
As stated, the partition coefficient is the ratio of concentrations of un-ionized compound between the two solutions. To measure the partition coefficient of ionizable solutes, the pH of the aqueous phase is adjusted such that the predominant form of the compound is un-ionized. The logarithm of the ratio of the concentrations of un-ionized solute in the solvents is called log P and calculated as follows (see Equation 1):10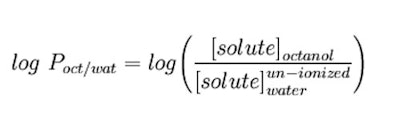 The Bottom Line
The Bottom LineSo, what does all this mean to a formulator? There are several take-away ideas. First, the partition of materials into the phases of an emulsion is a complicated situation, and not only depends upon the oil and water chosen, but also is affected by other additives including the emulsifier used to make the product. Thus, stability testing is critical.
The second consideration is: Where do the water-soluble actives go when the water in an emulsion evaporates? Think of a sunscreen with both water-soluble and oil-soluble actives. How uniform is the film left behind, and how do remaining actives affect film formation? A uniform film is required to obtain a high SPF. This means one must consider not only the partition coefficient in the emulsion, but also after the water evaporates.
All these parameters must be considered to create a successful product, and this is no easy task. In fact, it leads me to a comment I have offered before to the industry: “If we fully evaluated the interactions that occur in our formulations, we would have enough work for a doctorate for each product we do.” Happy formulating.









