Liquid-liquid extraction, also known as solvent extraction or partitioning, is a method used to separate compounds based on their relative partitioning in two different immiscible liquids. It is the extraction of a substance from one liquid phase into another liquid phase; in other words, it is the separation of a substance from a mixture by preferentially dissolving that substance in a suitable solvent. By this process, a soluble compound is separated from an insoluble matrix.
The present article deals with the ability to extract actives from botanical compounds or other mixtures in amphiphilic silicone compounds having different partition coefficients, which are obtained by altering the ratio of oil-soluble, water-soluble and fluoro-soluble groups on the silicone molecule. The ratio of the various groups determines the ability of the molecule to partition in different phases as well as what will be extracted into each phase.
Group Opposites
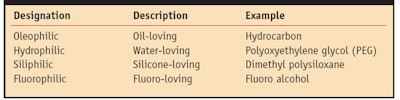
Before delving into the extraction process, a review of the mechanisms involved would be helpful. The concept of hydrophobic and hydrophilic is relatively easily understood; simply put, things that are hydrophobic are water-hating materials and things that are hydrophilic are water-loving. This simple definition works well with oil and water systems. Oils are hydrophobic, and water-soluble materials are hydrophilic. The other side of the coin is that oils are oleophilic or oil-loving, and water-soluble materials are oleophobic or oil-hating. As is normally the case, however, the world is more complicated. Silicone oil is neither oil-soluble nor water-soluble.
Silicone oil is in fact hydrophobic (water-hating) and oleophobic (oil-hating); however, defining a phase by its incompatibilities is not technically appealing—silicone oil is thus siliphilic or silicone-loving. Table 1 shows these group opposites. Note that hydrophobic materials can be either oleophilic or siliphilic; oleophobic materials may be either hydrophilic or siliphilic; and siliphobic materials may be either oleophilic or hydrophilic. Further, there are four types of groups that are mutually insoluble (see Table 2).
Silicone polymers can be designed to include two, three and all four of these types of groups in a single molecule, and it is this ability to modify silicone polymers to different partition coefficients that enables chemists to perform the extraction. As noted, the ratio of the groups in the polymer determines not only if the resulting polymer is soluble in oil, water, fluoro or silicone, but also into which phase the different components of an active can be extracted.
The term amphiphilic refers to a compound that possesses at least two groups that, if present in pure forms rather than in a compound, would be insoluble in one other.1 In amphiphilic compounds, the groups most commonly encountered are oil and water. When a molecule contains groups that make it an amphiphile, the resulting product is surface active and a wide range of such compounds can be found in nature as well as synthesized in the lab.
Partition Coefficient
The partition (P) or distribution (D) coefficient is the ratio of concentrations of a compound subjected to extraction in the two phases of a mixture of two immiscible solvents at equilibrium.2 Hence, these coefficients are a measure of the differential solubility of a compound present in a material subjected to extraction when exposed to two solvents. Typically one of the solvents chosen is water while the second is of a hydrophobic nature, such as octanol.3 Therefore, both the partition and distribution coefficient are measures of the hydrophilic or hydrophobic properties of a given compound.
The phrase partition coefficient refers to the equilibrium distribution of the solute between the two immiscible solvent phases. For example, when an organic compound is placed into a solvent mixture of ether and water, it separates or partitions into the water and ether phases. At equilibrium, the ratio of the concentrations of the organic solute in each of the solvent layers is its partition coefficient.
The extraction of actives from botanicals by amphiphilic silicone compounds with different partition coefficients extends the potential for extraction to silicone- and fluoro-soluble materials. This allows for a more efficient and specific extraction of the desired active-containing fractions from plant materials in a carrier that is more skin substantive. In addition, such extraction better retains the dermatocosmetically active constituents.
Plants were the first and remain one of the most important sources of active compounds for medicinal and cosmetic products. The many skin benefits and healing properties associated with topically applied botanical extracts include the reduction of transepidermal water loss, improved barrier function, increased moisturization, decreased inflammation and/or reddening, free radical scavenging and protection from UV-induced photodamage. The benefit(s) to be realized from using botanically derived materials, particularly in the dermato-pharmaceutical arts, therefore requires isolating and concentrating specific actives.
Throughout the centuries, a variety of techniques have been employed to extract active ingredients from botanicals. Solvent-based extraction is one such methodology. After segregating the plant material into its constituent parts (e.g., leaves, stems, fruits, branches, roots, etc.), the part with the desired active(s) is chosen, macerated (or similarly processed) and placed into the solvent. Plant materials that are soluble in the solvent are dissolved, leaving insoluble materials behind.
The extraction of actives from botanicals by amphiphilic silicone compounds with different partition coefficients extends the potential for extraction to silicone- and fluoro-soluble materials.
Solvent-based extraction systems are recognized as having at least two important limitations: First, they are able to extract only those materials that are soluble in the chosen solvent; second, because many solvents lack selectivity, compounds other than those desired are extracted. Not only can this dilute the purity of the desired active compound, it can also negatively impact the performance benefits of the extract.
For water-soluble actives, a number of extraction techniques are known. For example, hot water extraction removes water-soluble materials, leaving behind materials that are oil-soluble or insoluble in either water or oil. Tinctures, produced using hydro-alcoholic solutions, are another extraction vehicle well-known to skilled artisans. The inclusion of alcohol alters the polarity of water, producing different and marginally more efficient (e.g., higher yield) extracts than water or alcohol alone. Propylene glycol is yet another commonly used vehicle to extract water- and alcohol-soluble materials.
Extraction is a well-known technique for separating chemical constituents. It is a process by which a solute is extracted from a first solvent into a second solvent, whereby the two solvents are immiscible. One extraction methodology common in organic chemistry involves combining water and diethyl ether in a separatory funnel. Since ether and water do not mix, without agitation, they rapidly separate into two phases or layers.
Extraction procedures of the types described are, by their nature, inefficient in terms of maximizing the yield of a desired organic component. For example, take a 1,000-mg sample of a botanical material having an ether/water partition coefficient of 80; after a first pass in a separatory funnel, 800 mg of the botanical will be extracted into the ether phase. In order to recover still more botanical material from the remaining 200 mg (e.g., in the water phase), the separation procedure is repeated, each time with fresh ether solvent. The second pass is expected to remove an additional 140 mg, or 80% of the ether-soluble botanical components. However, even with multiple repetitions, some amount eludes extraction.
Adaptable Extraction
Polar (i.e., hydrophilic) materials extract different classes of compounds from botanical materials than do solvents having different polarities. The ability to extract compounds from botanical compounds over a wide range of polarities is highly desirable since some interesting actives are oil-soluble, others are water-soluble and still others have solubility in silicone solvents. Therefore, a series of amphiphilic silicone polymer reagents was developed for the extraction of compounds from botanicals (i.e., plant materials) whose efficacy can be targeted to various compounds according to the amounts of silicone-soluble, oil-soluble, fluoro-soluble and water-soluble groups attached to the silicone molecule. As noted, by altering the ratio of each group relative to the others, a wide range of partition coefficients can be created, thereby effectively extracting materials from plants. Solvents used to extract compounds from plant and animal tissues and preparing drugs is referred to as menstruum.
The extraction of broccoli sprouts using solvents of different polarities provides insight into the results obtained by extracting one material using different solvents. For this study, broccoli sprouts first were obtaineda. Broccoli sprouts are a class of materials that have been reported to protect against UV radiation-induced skin damage, including cancer. One constituent in broccoli sprouts of particular interest is sulforaphane (C6H11NOS2) (see Figure 1), a break down product of glucosinolate glucoraphanin.
Sulforaphane is also an isothiocyanate, more particularly 4-methylsulfinylbutyl isothiocyanate and (-)-1-isothiocyanato-4(R)-(methylsulfinyl) butane. As will be shown, four different silicone polymer menstrua extract four different active fractions having four different FTIR spectra. Spectral subtraction of the menstrua allows for the identification of groups present in the extracted materials. Menstrua based on the partition coefficient technology in the described invention allow for the rapid screening and effective extraction of this class of materials from broccoli sprouts as well as other cruciferous materials.
The use of silicone-based compounds for the extraction of different fractions of materials from plants not only produces the desired actives in a menstruum that provides for extraction, but also imparts desired aesthetics.
Extraction Procedure
Dried and crushed broccoli was extracted with each of the menstruum materials (see Figure 2a–d) one at a time. The plant material was then placed in a filter bag having a pore size of 100 microns. The amount of plant material was 5% by weight and the silicone-based menstruum comprised 95% of the weight. The menstruum was added to a recirculaton vessel, heated to between 60°C and 90°C, and recirculated through the filter bag for a period of 12 hr and 24 hr to remove solid plant material. The progress of the extract was monitored by FTIR, as described above.
Results
Of the total amount of plant material subjected to extraction, 12.1% of the material went into the various solvents, leaving 87.9% of the plant material behind as insoluble solvent (data not shown).
Oil-soluble silicone extract: The silicone menstruum having alkyl-soluble groups extracted 3% of the actives. Based on FTIR, spectral subtractions were esters, unsaturated compounds, some amine groups and some ketones. The extract changed in color from water-white to pale yellow. The skin feel of the product was cosmetically elegant and provided a smooth feel with good glide and spread. The extract in this example could be used as-is—i.e. in the menstruum without further modification, as well as in a topical emulsion system.
Silicone-soluble extract: This menstruum of broccoli from the silicone-soluble groups extracted 0.9% of the plant material. The functional groups present on the FTIR after spectral subtraction were primarily alcohols and ketones. The extract had no color change and the skin feel was cosmetically appealing, providing a dry, powdery feel with outstanding spread. Again, the extract could be used as-is—i.e. in the menstruum without further modification, or incorporated into topical emulsion systems by those skilled in the art. This fraction, unlike the starting material, also had distinct UV spectra.
Water-soluble silicone extract: This menstruum of broccoli, rich in water-soluble groups, was the most effective of the four examples in extracting material—7% by weight was extracted. The functional groups present on the FTIR after spectral subtraction were alcohols, some esters, a large amount of unsaturated compounds and some ketones. The extract changed in color from water-white to an intense yellow. The skin feel of the product was cosmetically appealing, providing a smooth feel with outstanding glide and spread. This extract could be used as-is or combined into water. This extract did have a noticeable sulfur smell, which may be an indication of sulforaphane being present.
Fluoro-soluble silicone extract: This menstruum of broccoli, rich in fluoro-soluble groups, extracted 1.2% of the plant material. The functional groups present on the FTIR after spectral subtraction were esters and unsaturated compounds. The extract changed in color from a water white to pale yellow. The skin feel of the product was also cosmetically appealing, providing a smooth feel with outstanding spread and waterproofing properties.
Conclusion
The use of silicone-based compounds for the extraction of different fractions of materials from plants not only produces the desired actives in a menstruum that provides for extraction, but also imparts desired aesthetics. The cosmetic chemist can therefore use a variety of new extraction solvents beyond typical water- or propylene glycol-based systems to develop formulas with different extracted actives.
References
Send e-mail to [email protected].
- Wikipedia website, available at: http://en.wikipedia.org/wiki/Liquid-liquid_extraction (Accessed May 6, 2010)
- A Leo, C Hansch and D Elkins, Partition coefficients and their uses, Chem Rev 71(6) 525–616 (1971)
- J Sangster, Octanol-water Partition Coefficients: Fundamentals and Physical Chemistry, vol 2, Wiley Series in Solution Chemistry, John Wiley & Sons Ltd, Chichester (1997) p 178