Fatty acid bile acid conjugates (FABACs) are a family of small synthetic molecules that initially were developed as oral drugs to reduce fat build-up and accumulation in the liver. The structure-activity rationale is that the saturated fatty acid acts as a cholesterol solubilizing agent while the bile acid acts as a vehicle to enable secretion into bile and penetrate into the enterohepatic circulation. The amide bond further enhances stability against intestinal degradation.1 In the skin, however, cholesterol metabolism differs dramatically. Skin renewal is maintained by controlling the balance between proliferation, differentiation and apoptosis of epidermal cells,2 and it has been shown that this program of epidermal differentiation in keratinocytes is altered when cholesterol-enriched domains in the plasma membrane are disrupted.
Leveraging the innovation of FABACs in health care, the authors developed a specific FABACa based on a cholesterol-solubilizing moiety, i.e., saturated fatty acid, and a bile acid (cholic acid) as the vehicle to enable secretion into bile and entry into the enterohepatic circulation for potential skin benefits.3, 4 This compound was chosen for its relatively low molecular weight and lipophilicity, allowing it to penetrate skin, affect cholesterol on the cell membrane level and facilitate other mechanisms. Previous proteomic data has proven the activities of FABACs5-7 in enhancing ATP-binding cassette (ABCA1) cholesterol transporter and competitively inhibiting stearoyl-CoA desaturase (SCD1) enzyme. Therefore, it was hypothesized that the developed FABAC would affect skin in similar ways.
In this paper, the mechanisms of ABCA1 cholesterol transporter and SCD1 enzyme in the skin are detailed first, highlighting the structure-activity relationships (SARs) involved. Following this, in vitro screenings of the FABACa active are described; screenings determined the level that activity was occurring. Interestingly, compiled results suggest activities comparable to retinoic acid—the only drug currently prescribed for skin aging and known for anti-acne effects (see Isaacman et al. article for more on this ingredient). However, retinoic acid acts through nuclear receptors, whereas the new FABAC is believed to act on cellular membrane transporters and competitively inhibit enzymes by depleting cholesterol from the membrane, thereby changing membrane fluidity and the exposure of membrane-anchored receptors. This milder yet effective mode of action is an attractive option due to its larger margin of safety.
Cholesterol in Skin
The skin is a site of active lipid synthesis. In the stratum corneum, aliphatic lipids are synthesized de novo in the epidermis via phospholipids, and cholesterol is synthesized from acetate within hours after induction (see Figure 1); cholesterol esters are produced three to seven days later. The skin’s lipid profile affects its ability to serve key functions, such as acting as a barrier against insult and preventing water loss from the body.
Cholesterol is the second most abundant lipid in the stratum corneum after ceramides—which account for up to 50% w/w of total intercellular lipids. Cholesterol is known to promote the intermixing of different lipid species and to regulate their thermodynamic phase behavior. Cholesterol esters, i.e., the next step in the synthesis chain, contain unsaturated and saturated diacyl chains that contribute to the stratum corneum’s stability and fluidity, and promote its liquid condensed state. In these capacities, both cholesterol and its esters serve a fundamental role in skin’s lateral lipid organization, and their ratio controls skin barrier properties.8
Since skin stem cells undergo terminal differentiation, and skin acts in some aspects as a “separate entity,” as several compartments in the skin are not in equilibrium with the body’s circulation, and, therefore, it fulfills its own needs from the blood. Further, the metabolic pathways of skin lipids are different from the pathways in internal organs and blood; consequently, there is no correlation between the cholesterol levels in blood and skin.
The skin barrier renewal process involves the generation and secretion of lamellar bodies or granules from keratinocytes into the extracellular matrix (ECM), which requires utilizing a battery of enzymes.9 These lamellar bodies contain lipid precursors such as glucosylceramides, cholesterol, glycerophospholipids and sphingomyelin, as well as catabolic enzymes such as proteases, lipases, acid phosphatase and glucosidases. Such lipid precursors are metabolized in the ECM and fused end-to-end, forming progressively elongated membrane sheets—the intercellular lipid lamellar structure of the stratum corneum.
Under basal conditions, lamellar body secretion is relatively slow and corresponds to the kinetics demonstrated by Hedberg and Wertz.10 However, when acute insult to the barrier is applied, this process is accelerated to promote a sequence of recovery that is achieved after 72 hr in young skin (i.e., 20s/30s). This sequence includes increases in cholesterol, free fatty acids and ceramide synthesis, all of which are restricted to the underlying epidermis at the injured site and dependent upon a prior up-regulation of mRNA encoding to synthesize anabolic enzymes. Since the synthesis of each of these key lipids is essential for maintaining normal barrier properties, inhibiting their synthesis may lead to abnormalities and an impaired barrier that is more permeable.
Cholesterol Regulation, ABCA1 and SCD1
Keratinocytes require abundant amounts of cholesterol for maintaining a strong barrier and controlling cutaneous permeability; hence, the regulation of cholesterol homeostasis in the skin is of great importance. ABCA1 plays a pivotal role for cholesterol efflux. It regulates cholesterol levels by promoting the transport of cholesterol and phospholipids across cell membranes. Jiang et al.11 demonstrated the expression of ABCA1 in human keratinocytes and murine epidermis, confirming its localization both in the outer epidermis, i.e., stratum corneum and stratum granulosum, and lower compartments—including the stratum spinosum and stratum basale. The activation of ABCA1 was shown to lead to the activation of the retinoid X receptor in keratinocytes and in macrophages.12
Jiang et al. also demonstrated that acute disruption of the barrier by tape-stripping or the application of acetone increases the synthesis of cholesterol and suppresses the expression of ABCA1 transporter; that way, cholesterol remains local and is available for rapid barrier repair. Conversely, facilitating ABCA1 activity results in transport of cholesterol into the cell, reducing the presence of cholesterol in the keratinocyte membrane and stratum corneum. This suggests the ABCA1 transporter may be linked to keratinocyte differentiation.
In relation, studies13 point toward a cascade connection between stearoyl-CoA desaturase (SCD1) enzyme inhibition and ABCA1 activation in skin. Mice deficient in SCD1 demonstrated sebaceous gland atrophy, depletion of sebaceous lipids, dry skin and alopecia. Interestingly, researchers suggest that the deletion of SCD1 and resulting reduction in sebaceous lipids may be of value in the treatment of Acne vulgaris, which is associated with increased sebaceous gland activity.
Cholesterol and Lipid Rafts
A closer investigation of cholesterol points to its indirect role in controlling cell cycles. It accomplishes this via receptors and transporters at the cell membrane, affecting the organization of protein-binding functions, in turn changing the protein conformation and interacting with nearby clusters. The plasma membrane of cells is made up of a combination of glycosphingolipids and protein receptors organized in glycolipoprotein micro-domains referred to as lipid rafts (see Figure 2). These specialized entities compartmentalize cellular processes by organizing the assembly of signaling molecules, influencing membrane fluidity and trafficking membrane proteins. Lipid rafts are more ordered and tightly packed than the surrounding and relatively fluid bilayer but float freely in the membrane bilayer. They have been described as small (10-200 nm), heterogeneous, highly dynamic, sterol and lipid enriched domains. Further, small rafts can sometimes be stabilized to form larger platforms through protein-protein and protein-lipid interactions.14
One key difference between lipid rafts and the plasma membrane from which they are derived is the lipid composition. Research has shown15 that lipid rafts generally contain three to five times the amount of cholesterol found in the surrounding bilayer. Cholesterol interacts preferentially, although not exclusively, with sphingolipids due to their structure and the saturation of the hydrocarbon chains. So although not all of the phospholipids within the raft are fully saturated, the hydrophobic chains of the lipids contained in the rafts are more saturated and tightly packed than the surrounding bilayer. Cholesterol can be viewed as the dynamic glue that holds the raft together.
Due to the rigid nature of the sterol group, cholesterol partitions into the lipid rafts where the acyl chains of lipids tend to be in a less fluid state. One important property of membrane lipids is their amphiphilic character—having a polar, hydrophilic head group and a non-polar, hydrophobic region. Cholesterol has the ability to pack in between the lipids in rafts, serving as a molecular spacer and filling any voids between associated sphingolipids. The depletion of cholesterol from lipid rafts has been shown to change their organization and affect keratinocyte differentiation. It was demonstrated, for example, that when lipid rafts were treated with low concentrations of methyl-beta cyclodextrin, which entrapped cholesterol and removed it from the raft, a significant decrease in keratin 1 and 10 early differentiation markers was observed.16
Materials and Methods
Based on the biology and SARs, as stated previously, it was hypothesized that the specified FABAC would enhance ABCA1 cholesterol transporter and competitively inhibit SCD1 enzyme. Thus, its effects were assessed in vitro via a gene expression assay, described here.
Full thickness model: Gene expression was measured in a full-thickness skin culture modelb. The FABACa ingredient was applied to the surface of each test culture at a concentration of 0.5% and collected 24 hr post-application. Cultures treated with 10 µL 100% DMSO served as the vehicle control group. Tissues were collected in an RNA stabilization solutionc, and gene expression was analyzed using validated assaysd. A set of 94 genes known for functions in the skin were evaluated, including ABCA1 and SCD1 genes. Statistics were carried out using softwaree.
Results and Discussion
Of the 94 selected genes used in the panel, only two—keratin 1 and 10 (KRT 1 and 10)—demonstrated statistically significant deviations in expression (see Table 1). ABCA1 and SCD1 mRNas were not altered by the FABAC at the gene expression level as expected. However, considering the SARs and previous proteomic5-7 data, these findings, in fact, support the theory of effects being confined to the protein level. Further, the FABAC was speculated to be depleting cholesterol levels in lipid rafts. As noted, the depletion of cholesterol from lipid rafts was shown to change their organization and significantly decrease keratins 1 and 10; here, KRT 1 and 10 were reduced. To elucidate the receptors being affected further, additional studies are planned.
Keratins 1 and 10
To further understand the effects of the specified FABAC, consider the role of keratinocytes.17 Keratins are heteropolymeric structural proteins that form the intermediate filament. These filaments, along with actin microfilaments and microtubules, compose the cytoskeleton of epithelial cells. The intermediate filaments are assembled from keratin monomers, and the cornified envelope is assembled from a protein called involucrin, as well as others. Involucrin is synthesized in the stratum spinosum, where it is cross-linked by transglutaminase enzyme, which further stabilizes it. Thus, involucrin provides structural support to the cell and allows for resistance to microorganisms. Involucrin also binds to loricrin, another protein, and contributes to the formation of the cornified envelope.
Keratins 1 and 10 are heterodimers and major constituents of the intermediate filament cytoskeleton in the superbasal epidermis. Both keratins are expressed in the spinous and granular layers of the epidermis. Type II cytokeratins consist of basic or neutral proteins that are arranged in pairs of heterotypic keratin chains co-expressed during the differentiation of simple and stratified epithelial tissues. The down-regulation of keratins 1 and 10 has been associated with the up-regulation of involucrin, and both have been shown to be triggered by exposure to retinoic acid.18
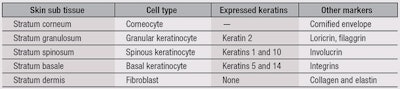
The up-regulation of involucrin production, as a result of the down-regulation of keratins 1 and 10, can therefore be explained as a compensation mechanism that allows the epidermis to maintain its integrity in spite of the attenuated differentiation. Table 2 cross-sections the normal human skin, outlining its layers, corresponding cell types, expressed keratins and other markers. It should be noted that the expression of keratins 1 and 10 is linked to the expression of involucrin and confined to the epidermal spinous layer—in which critical biochemical paths that determine the integrity of the barrier and its appearance are found.
Following the expression of keratins 1 and 10, pro-filaggrin, an important marker, is expressed and leads to the generation of filaggrin, a cationic protein specific to the stratum corneum. Taken together, in theory, the FABAC’s attenuation of keratinocyte differentiation and depletion of cholesterol from lipid rafts could lead to a compensation mechanism that results in accelerated proliferation and barrier rejuvenation, potentially renewing the skin and affecting its appearance. Additional studies are under way.
Retinoic Acid, and Keratins 1 and 10
Retinoic acid is the active form of vitamin A. The four most common indications for retinoids are: acne, wrinkles, photo-damaged skin and inheritable keratiopathies—however, the potential for teratogenic effects from the use of retinoids in women of children-bearing age is a key consideration.19 In addition, it is well-established that a common adverse effect from retinoid treatments is skin irritation, although the exact mechanism is not fully elucidated. The general hypothesis is that retinoids normalize keratinocyte differentiation; other possible mechanisms include the down-regulation of desmosomal proteins, anti-proliferative effects, regulating lipid synthesis, growth factors and cytokines.
Unlike the theoretical mechanism of the new FABACa, retinoids exert their effects entirely through nuclear receptors. There are at least six retinoic acid receptors belonging to two families: retinoic acid receptors (RARs) and retinoid X receptors (RXRs). Nuclear receptors expressed in keratinocytes include these two families of receptors, vitamin D3 receptor and thyroid hormone receptor. All of these can affect keratinocyte differentiation. For example, the RAR gamma receptor for retinoic acid plays an important role in the morphogenesis and differentiation of squamous epithelia.
Retinoic acid has been shown to inhibit the activity of keratinocyte transglutaminase and the formation of the cornified envelope. Similar to the FABACa, both keratins 1 and 10 were shown to be down-regulated in skin treated with retinoic acid in vitro and in vivo.20 Interestingly, however, not all differentiation markers are regulated by retinoic acid; involucrin, for example, is unaffected when cultured keratinocytes are treated by retinoic acid (see Isaacman et al. article for further discussion).
SCD1 and Anti-acne Potential
As noted previously, studies11 point to a connection between SCD1 enzyme inhibition and ABCA1 activation in skin; the deletion of SCD1 reduces secretion of sebaceous lipids, which may be of value in the treatment of acne. In relation, the indirect inhibition of SCD1 activity by FABACs does not appear to affect barrier integrity, unlike retinoic acid. This is believed to be a result of the compensation mechanism. While inhibition of SCD1 leads to an increase in ABCA1 transporter activity and the depletion of barrier cholesterol levels in keratinocytes, a compensatory enhanced production of cholesterol and ceramides may lead to a replenished barrier. This restored barrier is especially necessary in the case of reduced sebum secretion, which acts as a protecting layer.
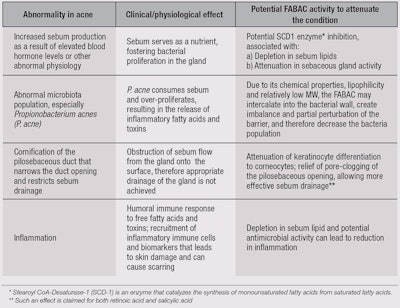
There are numerous factors for the initiation and progression of acne, and its severity can be related to the interplay between these factors. Table 3 and Figure 3 summarize the physiological conditions leading to acne and the anticipated activity of the specified FABACa based on its structure, SARs with similar FABACs, and data generated from the gene expression study.
Practical Applications
The presented active has potential for anti-aging and anti-acne applications, and the next steps are to study its behavior in topical formulations and acquire safety assessment data relevant to the skin. When considering a compound for potential biological activity, first-tier studies should seek to understand its mode of action (i.e., pharmacodynamics) and site of action—i.e., the skin sub-tissue and cellular, receptor and enzyme levels. Based on this screening, a hypothesis is drawn, which is the stage at which the present authors have arrived and at which this paper was written. Perhaps the most interesting aspect is that FABACs impart biological activity manifestations similar to those of a known drug but by affecting different cellular entities.
Summary
Here, the authors present the potential activity of a selected FABAC on skin. Initial in vitro screenings are presented, leading to a hypothetical suggested activity of the active. Based on screenings, SARs and the ingredient’s proposed mechanism of action, a hypothesis for its activity as an anti-aging and anti-acne active is drawn.
Interestingly, its suggested mechanism can be compared to that of retinoic acid, but while retinoic acid acts via the activation of nuclear receptors, the FABAC is thought to act on a cellular membrane transporter level and through competitive enzyme inhibition. Lastly, this apparently milder yet effective mode of action could translate to larger safety margins; when tested for cytotoxicity on a full thickness model, the FABAC demonstrated no significant changes in cellular viability up to levels of 2%.
Acknowledgments: Galderm Therapeutics, an Israeli start-up company, and Nava Dayan, PhD ([email protected]), collaborated on the present work; the authors are thankful to Prof. Philip W. Wertz, from the University of Iowa, for his critical review of this paper.
References
- I Goldiner et al, ABCA1 dependent but apo A-1 independent cholesterol efflux mediated by fatty acid-bile acid conjugates (FABA’s), J Biochem 396 526-536 (2006)
- J Nie, X Fu and W Han, Microenvironment-dependent homeostasis and differentiation of epidermal basal undifferentiated keratinocytes and their clinical applications in skin repair, J Eur Acad Dermatol Venereol 27(5) (2013) 531-535 (2013)
- T Gilat et al, Fatty acid bile acid conjugates (FABACs)—New molecules for the prevention of cholesterol crystallization in bile, Gut 48(1) 75-9 (Jan 2001)
- T Gilat et al, Arachidyl amido cholanoic acid (Aramchol) is a cholesterol solubilizer and prevents the formation of cholesterol gallstones in inbred mice, Lipids 36(10) 1135-40 (Oct 2001)
- US Pat 6384024B1, Bile salt conjugates, assigned to T Gilat (May 7, 2002)
- US Pat 6395722B2, Fatty acid derivatives of bile acids and bile acid derivatives, assigned to T Gilat (May 28, 2002)
- US Pat 6589946B2, Bile salt conjugates, assigned to T Gilat (Jul 8, 2003)
- I Kravchenko, Y Boyko, N Novikova, A Egorova and S Andronati, Influence of cholesterol and its esters on skin penetration in vivo and in vitro in rats and mice, Ukrainica Bioorganica Acta 1 (2011) 17-21
- MR Prausnitz et al, Skin barrier and transdermal drug delivery, available at drugdelivery.chbe.gatech.edu/Papers/2012/Prausnitz%20Derm%20Book%20Chapter%202012.pdf (Accessed Mar 21, 2014)
- CL Hedberg, PW Wertz and DT Downing, The nonpolar lipids of pig epidermis, J Inves Derm 90 225–229 (1988)
- YJ Jiang, B Lu, PM Elias and KR Feingold, Regulation of ABCA1 expression in human keratinocytes and murine epidermis, J Lipid Res 47 2248-2258 (2006)
- P Costet et al, Retinoic acid receptor-mediated iInduction of ABCA1 in macrophages, Mol Cell Biol 23 (21) 7756-7766 (2003)
- Ibid Ref 10
- S Lambert, R Gniadecki, and Y Poumay, Cholesterol and lipid rafts as regulators of signaling through the EGF receptor in keratinocytes, Open Dermatology J 3 151-158 (2009)
- LJ Pike, Lipid rafts, bringing order to chaos, J Lipid Res 44, 655-667 (Apr 2003)
- F Sporl et al, Real-time monitoring of membrane cholesterol reveals new insights into epidermal differentiation, J Invest Derm 130(5) 1268-1278 (2010)
- RL Eckert and ER Rorke, Molecular biology of keratinocytes differentiation, Environ Health Perspect 80 109-116 (1989)
- Y Poumay, F Herphelin, P Smits, IY De Potter and MR Pittelkow, High-cell density phorbol ester and retinoic acid upregulate involucrin and downregulate suprabasal keratin 10 in autocrine cultures of human epidermal keratinocytes, Mol Cell Biol Res Commun 2(2) 138-144 (1999)
- C Fisher, M Blumberg and M Tomic-Conic, Retinoid receptors and keratinocytes, Crit Rev Oral Biol Med 6(4) 284-296 (1995)
- H Torma, Regulation of keratin expression by retinoids, Dermato-Endocrinology 3:3 136-140 (2011)