The skin and mucous membranes of healthy individuals maintain normal functions with naturally occurring microorganisms on them. The numbers and types of these microorganisms depend on moisture level, pH, nutrient availability, the presence or absence of inhibitory materials and the immunological tolerance of different sites of the body. Microorganisms generally found on skin, on mucous membranes and in the gastrointestinal (GI) tract of healthy individuals constitute the normal microflora, meaning they are normally present and do not cause problems in healthy individuals. People would have continual microbial infections—boils, abscesses, inflammation, diarrhea and intestinal gas/bloating—if they did not live in harmony with their normal microflora.
For more than 50 years, it has been widely believed that the presence of microorganisms on the skin is part of a natural defense because the normal microflora helps protect against pathogenic microorganisms. This dogma has been accepted without a great deal of scientific support. Billions of bacteria inhabit the GI tract. The mucous membranes of the nose, mouth and vagina may have large numbers of microorganisms. There are relatively few reported studies directed at understanding the benefits of microorganisms normally present compared with the number of studies conducted to understand mechanisms by which pathogenic microorganisms cause infections, or to determine which antimicrobial agent(s) are most effective in treatment.
In recent years, there has been a dramatic increase in the popularity of probiotics in the Unites States. Probiotics are dietary supplements containing potentially beneficial bacteria or yeasts. These products generally have been marketed as food supplements and as dairy products. Promotion of probiotics is based on clinical studies demonstrating the benefits to intestinal health and laboratory studies demonstrating mechanisms by which probiotics achieve those benefits. The laboratory studies indicate that these microorganisms and/or their metabolites interact with intestinal epithelial cells to prevent adherence of undesirable bacteria and to down-regulate the intestinal immune system.
It is likely that the mechanisms by which probiotics affect mammalian cells are similar to mechanisms used by normal microflora in other parts of the human body. The goals of this article are to introduce readers to the normal microflora in several regions of the body, with emphasis on the skin microflora; to discuss probiotics and touch on their therapeutic activity and cellular microbiology; and to show some similarities between probiotics and skin microflora. It is hoped that this information will serve as the basis for studies to better understand mechanisms by which the human normal microflora maintains homeostasis with epithelial and mucosal cells so that formulators can apply this information to personal care products designed to maintain the health and beauty of the skin.
The Paradox
It is amazing that billions of bacteria inhabit an individual’s GI tract and high concentrations of microorganisms reside on specific skin sites and on mucosal surfaces, yet people are neither sick nor covered with infections. The intriguing question is: Why not?
Normal Microflora
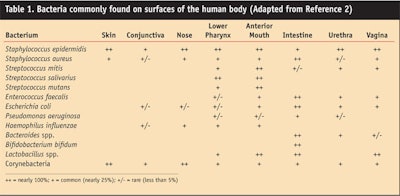
The skin, mucous membranes and GI tract are constantly in contact with the environment and they become colonized by bacteria, yeast, fungi and viruses. In healthy individuals, the types of microorganisms generally colonizing skin and mucous membranes are a few yeasts and fungi, but bacteria predominate. Aly and Maibach1 investigated the aerobic microflora of the axilla, groin, finger web and toe web. They found average counts per square centimeter of 1.3 x 107 for nonlipophilic diphtheroids, 3.0 x 106 for lipophilic diphtheroids, 1.3 x 105 for micrococci and 8.6 x 103 for Staphylococcus aureus. Of the four sites tested, the axilla had the highest average number of Gram-negative rods (3.8 x 102). Todor2 recently published more extensive information on the predominant bacterial microflora of humans (see Table 1).
Bacteria, yeasts and fungi have adaptive survival strategies that enable them to deal with adverse conditions.3 This is why microorganisms can still cause problems, in spite of all the body’s natural defenses. The interaction between individuals and their resident microflora is thought to be dynamic and generally beneficial:
•Enteric bacteria produce and secrete vitamins K and B12.
•Lactic acid bacteria produce and secrete B vitamins.
•Resident microorganisms prevent colonization by other bacteria and pathogens by competing for attachment sites and essential nutrients, by producing fatty acids, by lowering the pH in microenvironments, by producing peroxides and specific bacteriocins that kill or prevent growth of other bacteria, and by stimulating epithelial cells to produce defensins, cathelicidins and other small antimicrobial peptides.
•Intestinal microorganisms stimulate the development of certain tissues (e.g., caecum and Peyer’s patches in the GI tract).2
•Microorganisms stimulate the immune system (skin immune system and gastric associated lymphoid tissue) and induce the production of antibodies.
Probiotics
Probiotics are living microorganisms. They are considered to be live food ingredients that are beneficial to health. Probiotics are consumed to normalize the intestinal microflora and protect the intestinal mucosa from harmful microorganisms.
In recent years, the health benefits of consuming cultured dairy products that contain probiotics have been promoted in television commercials, magazine ads and health food stores. Products containing probiotics include acidophilus milk, yogurt, frozen yogurt, and various acidophilus tablets/capsules, foods and drinks. These products may contain lactic acid bacteria including Lactobacillus acidophilus, Lactobacillus strain GG, Lactobacillus reuterii, L. bulgaricus, and Streptococcus thermophilus, bifidobacteria (Bifidobacterium bifidum, B. longum, B. infantus and Bifidus regularis), and/or yeast (Saccharomyces boulardii).
Probiotics in the intestines: In the GI tract, the health benefits of probiotics are believed to result from several mechanisms.
•Probiotics are believed to protect intestinal epithelial cells by competing with pathogens for mucosal adherence sites. The first step in infection is the attachment of a microorganism to a target cell. Adherence by probiotics blocks pathogens from attaching to their target cells so they are stopped before they can initiate an infection. This is known as colonization resistance.
•Probiotic microorganisms are believed to compete with other microorganisms for nutrients in the GI tract.
•Probiotics work in situ to promote immunological quiescence by modulating dendritic cells to enhance the release of interleukin-10.4
•Probiotics may stimulate epithelial cells to produce antimicrobial peptides.5
•Probiotics may up-regulate production of cathelicidins by intestinal epithelial cells.6 Cathelicidins are small cationic peptides that have broad specificity against bacteria, fungi, parasites and viruses. They are thought to kill bacteria by disrupting cell membrane integrity and/or by blocking protein synthesis.7
For more on the therapeutic activity of probiotics, see the author’s companion article, Therapeutic Activity of Probiotics, on Page 20.
Prebiotics
Prebiotics are nutrients that favor the growth of probiotic microorganisms. Prebiotics include fructose-containing polysaccharides, such as inulin, and often enzymes (i.e., proteases, cellulase, amylases, ß-galactosidase) that help break down foods to aid growth of probiotic microorganisms and digestion. Fructose-containing polysaccharides favor water retention in the colon and fermentation by probiotic microorganisms that have enzymes to utilize these polysaccharides.
Lactic acid is the most common fermentation end-product produced by these organisms. It helps lower the intestinal pH and this, in turn, makes it more difficult for non-acid-tolerant bacteria to grow. Consuming probiotics (especially with prebiotics) is thought to help increase the number of lactic acid bacteria and bifidobacteria in the colon and decrease less desirable bacteria (i.e., bacteroides, clostridia, coliforms) that may colonize intestinal epithelial surfaces and initiate inflammation or gastric immunological reactions. Replacement of these undesirable bacteria by probiotic microorganisms is believed to restore and/or maintain intestinal homeostasis.
Skin Microflora
The skin microflora includes propionibacteria, staphylococci, Malassezia spp., micrococci and corynebacteria.8
Health benefits for the skin: As noted earlier, microorganisms residing on the skin and mucous membranes may have desirable effects on human health:
•Growth of specific microorganisms on skin and mucous membranes lowers the pH, which makes it more difficult for competing microorganisms—including pathogens—to grow.
•Organisms comprising the skin microflora compete with potential pathogens for nutrients, which makes it more difficult for them to become established and cause infections.
•Attachment of the microflora to receptor sites may block potentially undesirable microorganisms (i.e., pathogens) from attaching to cells to initiate an infection.
•Association of microorganisms with immunologically competent cells in the skin, such as keratinocytes and Langerhans cells, may stimulate the skin immune system (SIS) so that the SIS is in a state of readiness when stimulated by potentially harmful microorganisms. Cathelicidin and human β-defensin-2 are induced by keratinocytes in response to infection.9-10
The protection afforded by helping create the acid mantle condition of the skin and lowering skin surface pH may be a double-edge sword. Propionibacteria and staphylococci produce lipases that release free fatty acids from tri-
glycerides in sebum. They also produce short-chain free-fatty acids (lactic and propionic acids) and vasoactive amines (histamine and tryptamine).11 Areas of high bacterial density on the skin include the T-zone and the nasolabial folds at the sides of the nose.
In the writer’s opinion, the presence of high levels of microorganisms contributes to the sensitivity of facial areas—especially the T-zone—because the antigenic components of bacterial cell walls and metabolic products including short-chain fatty acids and vasoactive amines may induce a subclinical state of inflammation. Skin in that subclinical state is more prone to irritation when lactic acid is applied or when consumers use products containing fragrances, preservatives or other potentially irritating chemicals. Studies are needed to determine whether there is a correlation between the density of staphylococci and propionibacteria and the facial stinging responses of different individuals.
Probiotics in a topical product: In 2005, Sullivan et al.12 patented a skin treatment method with Lactobacillus extract for stimulation of β-defensins in skin cells. Stimulation of cationic peptides is a way to increase the skin’s natural defenses against infection with microorganisms, as in the treatment of acne, or to lower microbial density on sensitive areas of the skin (i.e., the face) to make products less irritating.
Although aqueous products in multiple-use containers require a preservative system that would kill unprotected probiotic microorganisms, this problem could be circumvented by use of unit-dose packaging containing probiotic microorganisms such as the Lactobacillus spp, by protecting the beneficial bacteria by encapsulation, or by use of alternate means of preservation including refrigeration of products with short shelf-lives.
Also, it is possible that dead microorganisms or extracts may retain some of the benefits of living cells. For example, the dead microorganism may still be able to block receptor sites or stimulate epithelial cells to release antimicrobial cationic peptides.12 Formulation with such materials would be much easier than formulating with living microorganisms in products.
Another approach would be to use prebiotics or nutrients that would select for desired microorganisms in target sites such as the skin, mouth, vagina or GI tract. Such an approach would seem to be working with Mother Nature to facilitate the growth of the normal microflora in sufficient numbers to help maintain homeostasis.
Similarities Between Probiotics and Skin Microflora
Although extensive studies have been performed to determine some of the mechanisms by which probiotic bacteria maintain intestinal homeostasis, there seems to have been few studies of this sort performed to assess the role played by normal human skin microflora in maintaining the healthful condition of the skin and mucous membranes. Nevertheless, sufficient evidence exists to suggest some similarities between probiotics and resident skin microflora, as follows:
•Generally, probiotics and skin microflora are Gram-positive bacteria.
•Probiotics and skin microflora are thought to compete with potentially harmful microorganisms for nutrients and to block receptor sites on target epithelial cells.
•Probiotics and skin microflora produce short-chain free-fatty acids (lactic and propionic acids) that help lower the pH of their milieu and make it more difficult for potentially harmful microorganisms to grow.
•Probiotics are reported to down-regulate inflammatory responses by producing non-inflammatory cytokines (IL-10) and reducing the release of nuclear factor κß (NF-κß) and inflammatory cytokines. This has not been established for the resident skin microflora but it would appear to be the case for normal individuals who have no obvious inflammatory condition even though they may have more than 106 bacteria/cm2 on some skin sites or on mucous membranes.
•Neither probiotics nor the skin microflora appear to induce apoptosis, which is a frequent cellular response to cellular injury following invasion by pathogenic bacteria.
Summary
There has been tremendous growth of yogurt- and probiotic-containing foods in recent years because consumers are finding that these products aid in normalizing intestinal conditions. Research on probiotics is demonstrating that the cross-talk between the bacteria and mammalian cells involves signal transduction that affects production of key signaling molecules, such as NF-κß, and effector molecules including heat shock proteins and cytokines.
There are some interesting parallels between resident skin microflora such as staphylococci and propionibacteria and probiotic bacteria in terms of how they may alter their microenvironment and how high densities of microorganisms associate with host cells without apparent inflammation or skin irritation.
Humans coexist with microorganisms. The time is right for considering how to make advantageous use of microorganisms. The skin treatment method with Lactobacillus extract for stimulation of β-defensins in skin cells illustrates that this approach has value. It is possible that probiotics and modulation of normal microflora will be the next frontier as formulators translate learnings from clinical studies to products that help maintain the health and beauty of skin.
References
1.R Aly and HI Maibach, Aerobic microbial flora of intertrigenous skin, Appl Environ Microbiol 33 97–100 (1997)
2.K Todar, The bacterial flora of humans, Todar’s Online Textbook of Bacteriology, Madison: University of Wisconsin (2007)
3.DS Orth, The effect of acid pH on microorganisms and survival strategies that permit growth in products, In Preservative-Free and Self-Preserving Cosmetics and Drugs. Principles and Practice, JJ Kabara and DS Orth, eds, New York: Marcel Dekker 15–44 (1997)
4.M Drakes, T Blanchard and S Czinn, Bacterial probiotic modulation of dendritic cells, Infect Immun 72 3299–3309 (2004)
5.J Wehkamp et al, NF-kb- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 19717: A novel effect of a probiotic bacterium, Infect Immun 10 5750–5758 (2004)
6.J Schauber et al, Expression of the cathlecidin LL-37 is modulated by short chain fatty acid in colonocytes: relevance of signaling pathways, Gut 52 735–741 (2003)
7.V Nizet and RL Gallo, Cathelicidins and innate defense against invasive bacterial infection, Scand J Infect Dis 35 670–676 (2003)
8.JJ Leyden, KJ McGinley, KM Nordstrom and GF Webster, Skin microflora, J Invest Dermatol 88 65s–72s (1987)
9.MH Braff, M Zaiou, J Fierer, V Nizet and RL Gallo, Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens, Infect Immun 73 6771–6781 (2005)
10.JGH Dinulos, L Mentele, LP Fredericks, BA Dale and GL Darmstadt, Keratinocyte expression of human β defensin 2 following bacterial infection: Role in cutaneous host defense, Clin Diag Lab Immun 10 161–166 (2003)
11.RP Allaker, J Greenman and RH Osborne, The production of inflammatory compounds by Propionibacterium acnes and other skin organisms, Br J Dermatol 117 175–183 (1987)
12.WO/2005/091933, Skin treatment method with Lactobacillus extract, M Sullivan, SF Schnittger, T Mammone and E Goyarts, assigned to E-L Management Corp (Oct 6, 2005)