Cosmetic chemists are all familiar with emulsions. We are all quite aware that oil and water don’t mix, so if both are in our formulation, an emulsifier is often added to maintain an emulsion.
An emulsion is a mixture of two phases that are immiscible in one another; most often oil (esters, hydrocarbons and silicone) and water, together with a surface active agent that allows for the preparation of a metastable product that, over time, will separate. Good emulsions are stable for a long time but thermodynamically, they will separate.
See related: Emulsion vs. Invert Emulsion
Emulsions
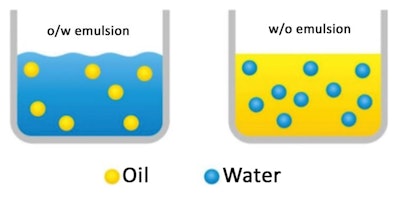
There are at least two immiscible phases in an emulsion. The phase listed first in the description, represented by the round spheres in Figure 1, is the internal or discontinuous phase. The phase listed second is the external or continuous phase;1 shown here are both oil-in-water (o/w) and water-in-oil (w/o) emulsions. Additional types include silicone-in-water (si/w) and water-in-silicone (w/si) emulsions, wherein silicone is the oil phase.
Figure 1. Depiction of an o/w and w/o emulsion2