Discovered in the 1990s, the RNA interference (RNAi) process has emerged as being important to gene expression modulation in eukaryotic cells. This post-transcriptional control mechanism is mediated by small endogenous noncoding RNAs (18-25 nucleotides) named microRNA (miRNA). These sequence-specific, post-transcriptional regulators bind to partially complementary sequences of their target messenger RNA (mRNA). This binding can lead to several outcomes, including the translation repression or degradation of the mRNA, which cause gene inactivation or gene silencing.
Generated by the ribonuclease III Dicer, miR, as found in the cytoplasm, consists of a mature miRNA strand and an opposite one, identified with an asterisk—i.e., miRNA*. When the regulatory pathway is initiated, the miRNA duplex (double-stranded) combines with an argonaute protein (RNase H enzymes) to form a multiprotein invalidation complex induced by RNA, named RNA-induced silencing complex (RISC). Argonaute proteins contain two domains: PAZ (i.e., binding to miRNAs) and PIWI (binding to target mRNA). During RISC formation, double-stranded miRNA becomes single stranded and mature miRNA guides RISC to its target mRNA.
As noted, several post-transcriptional regulation mechanisms are possible, such as the degradation or translation inhibition of the target mRNA. The mechanism expressed will depend on the argonaute protein that formed the RISC complex and the complementarity between the miRNA and its target.
The role miRNAs have in regulating gene expression is considered as important as transcription factors themselves. One miRNA can control more than 100 transcripts and one gene can also be regulated by several miRNAs. These miRNAs are involved in regulation processes such cell cycles, DNA repair systems, reactions to oxidative stress, apoptosis, etc., and each tissue has a specific miRNA expression profile.
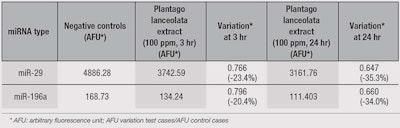
Recent studies have examined the role of specific miRNAs in the control of collagen-I, collagen-IV and elastin synthesis in the skin. Maurer et al.,1 Kwiecinski et al.2 and LI et al.3 demonstrated the importance of increasing miR-29 expression and the reduction of collagen-I and collagen-IV synthesis. Conversely, TGF-β leads to a reduction in miR-29 expression and an increase in collagen synthesis. Kashiyama et al.4 and Honda et al.5 indicated that miR-196a also suppressed collagen-I and collagen-III synthesis. Such a decrease in miR-29 production also increases elastin synthesis.6, 7
It appears that collagen-I, collagen-IV and elastin are partially controlled by several microRNAs, and when these microRNAs are moderately limited, it helps to boost collagen and elastin synthesis to improve the quality of the dermis. In parallel, Gabriely et al.8 and Ren et al.9 demonstrated that a decrease in the production of miR-21 contributes to the increase of TIMP production and consequently, to the reduction of MMP activity.
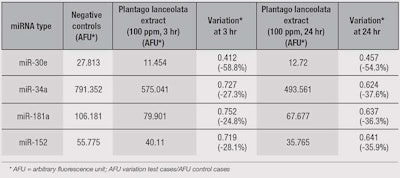
Other teams, including Khanna et al.10 and Mancini et al.,11 respectively demonstrated that the trio of miR-30e, miR-34a and miR-181a, and the duo of miR-152 and miR-181, participate in increasing senescent phenotypes in neurons and fibroblasts. Decreasing the number of these miRNA molecules also helped to prolong cell survival and reduce the appearance of senescent phenotypes. These studies demonstrate the importance of this miRNA-regulated pathway.
The present study examines the effects of an extract of Plantago lanceolata, derived from plant cell culture technology, in regulating the above mentioned microRNAs to fight signs of senescence. Preliminary results from anti-aging studies led to this particular ingredient’s selection.
Materials and Methods
MiRNA expression levels: The following method was used to measure the expression levels of all miRNAs studied. Fibroblasts were seeded in 35-mm dishes and cultured to confluence in their maintenance medium. Twenty-four hours before contact with an ethanolic extraction of lyophilized Plantago lanceolata cells, derived from a plant cell culture technology, the medium concentration in serum was lowered.
Cells were then incubated with 100 ppm of the Plantago lanceolata extract for either 3 hr or 24 hr. After incubation, the total RNA, including miRNAs, was extracted from the cells and the miRNA expression profile was determined on an miRNA test chipa and compared with a control. These results, described later, were further supported by the measurement of the targeted markers synthesis—i.e., collagens, elastin, MMPs, TIMPs, zona-occludens-1 and claudin-1—using using regular methods, described next.
Collagen-I: Human dermal fibroblasts (HDF) in culture were exposed to the Plantago lanceolata extract (100 ppm) for six days. Collagen-I synthesis was measured by photography after immunolabelling. Cellular nuclei were counted using a DNA stainb to standardize the data.
Collagen-IV: Abdominal skin explants from a 53-year-old woman were placed in appropriate medium and treated with either ~10 µL of a cream containing 330 ppm of the Plantago lanceolata extract, applied with small brush on ~0.5 cm2 of the skin explants, or a placebo cream as the control. These were applied every day for nine days. At the end of this contact period, sections of skin were taken and collagen-IV was labelled with a fluorescent antibody. Thirty pictures (n = 10 per case) were used to quantify and compare the respective quantities of collagen-IV.
Elastin: Abdominal skin explants from a 53-year-old woman underwent accelerated aging, per Oishi et al.,12 then a cream containing 330 ppm of the Plantago lanceolata extract or a placebo control was applied for nine days. The skin sections were analyzed under a microscope after immunolabelling of the elastin. Thirty pictures (n = 10 per case) were used to quantify and compare the respective quantities of elastin.
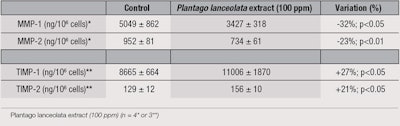
MMP and TIMP: HDFs were subjected to oxidative stress (H2O2), then MMP-1 and MMP-2 production were monitored by ELISA, as were TIMP-1 and TIMP-2 production—in the presence or absence of the studied extract (100 ppm).
Laminin: Human keratinocytes were placed in contact with the Plantago lanceolata extract (100 ppm) for three days, and the laminin content of the culture supernatants was determined using the ELISA method.
Keratinocyte differentiation speed: Human keratinocytes were placed in contact with the Plantago lanceolata extract (200 ppm) and the speed of differentiation was monitored for seven days using red oil dye specific to neutral lipids. Pictures (n = 15 per case) were used to quantify and compare the respective quantities of keratinocytes.
Zona-occludens-1 (ZO-1) and claudin-1: Human keratinocytes were placed in contact with the studied extract (100 ppm) for two days and immunolabelling was performed to enable the two cohesive elements of the upper portion of the epidermis to be monitored: ZO-1 and claudin-1.
Melanin and viscoelastic properties: Samples of stratum corneum were taken using adhesive discs and colored using the Fontana Masson method to reveal melanin content. Standardized photographs were also taken in vivo and analyzed to quantify residual pigmentation, expressed as a percentage of the overall non-pigmented area. In addition, viscoelastic properties including skin firmness and elasticityc, and thickness and densityd were measured in vivo after one month of application. Finally, skin smoothness was measured after two months of application using silicone replicas of the crow’s feet area.
Results and Discussion
Plantago lanceolata extract was found to reduce the levels of expression of miR-29 and miR-196a, both of which are known for exerting negative control on the synthesis of collagens and elastin. These observations were reinforced by decreases in the expression of miR-25 and miR-150, which also repress collagen and elastin synthesis (see Figure 1 and Table 1).
The results also were corroborated by the measurement of the targeted markers synthesis using regular methods. Collagen-1 increased by 385% (p < 0.01) (see Figure 2); collagen–IV increased 53% (p < 0.01); and elastin increased 76% (p < 0.01) (see Figure 3), in comparison with “aged” skin. Taken together, these results confirm the action of Plantago lanceolata extract on miRNAs involved in the neosynthesis of dermal macromolecules to counteract the loss of density and thickness of the dermis.
Further, the extract was shown to significantly decrease miR-21 expression (see Figure 4), which demonstrates the protection provided to dermal macromolecules. These observations were confirmed by the variation in quantities of MMP-1 and -2, and TIMP-1 and -2 in samples treated with the extract (see Table 2).
Plantago lanceolata extract also induced a decrease in the expression of miR-30e, miR-34a, miR-181a and miR-152 (see Table 3), which leads to a prolongation of cell survival and reduction in the appearance of senescent phenotypes.
These observations were supported by the measurement of targeted markers synthesis. In the presence of the extract, laminin production was higher (+46%, p < 0.01) than in the control. Taken with the results for collagen-IV, this shows the extract ensures that basal layer keratinocytes are more firmly anchored, helping to maintain the homeostasis of this cellular layer. The Plantago lanceolata extract also increased keratinocyte differentiation speed by +63% (p < 0.01), which contributes to epidermal barrier reinforcement (see Figure 5).
The extract also was found to promote the synthesis of ZO-1 (+42%, p < 0.01) and claudin-1 (+125%, p < 0.01), both of which are integral parts to tight junctions in the skin.
The entirety of these results indicate that Plantago lanceolata extract, by targeting the appropriate miRNAs, is effective at fighting chronological or induced epidermal aging, and in reducing the progression toward senescence. Additional in vivo studies confirmed the macro effects of these mechanisms observed at a micro level.
Viscoelastic properties were improved, with increases in firmness of 30.9% and elasticity of 22.6%, after one month of product application (p < 0.01, vs T0) (see Figure 6); as well as thickness and density, with increases of 4.8 µm (15%, p < 0.01) in the epidermis and 33 µm (3.6%, p < 0.01) in the dermis, also after one month of product application. Skin density also increased by 35% after one month (p < 0.01, vs T0), and skin smoothness was enhanced by 11.9% after two months (p < 0.01, vs T0). Finally, a 26% reduction in pigmentation, i.e., melanin, in age spots was observed with the extract versus the placebo, demonstrating control over melanocytic activities.
Conclusion
Recently, micro molecules of RNA, or miRNA, were discovered and found in higher quantities in aging and senescent cells. These molecules play a significant role in aging because they regulate the synthesis of proteins essential to maintaining tissue homeostasis.
Several types of miRNA have been identified according to their action on cellular senescence and on the destruction of the extracellular skin matrix, as presented here. These could act as new targets for the development of anti-aging actives, such as the Plantago lanceolata extract discussed here. However, the industry has only just begun to discover the mechanisms of miRNA, and more particularly, the molecules that can act on them to lead to significant advances in cosmetic science.
References
- B Maurer et al, MicroRNA-29, Arthritis & Rheum 62 1733-1743 (2010)
- M Kwiecinski, PLOS One 6 e24568 (2011)
- J Li, M Ghazwani, Y Zhang, J Lu, J Fan, CR Gandhi and S Li, J Hepatol 58 522-528 (2013)
- K Kashiyama et al, J Invest Dermatol 132 1597-1604 (2011)
- N Honda et al, J Immunol 188 3323-3331 (2012)
- XR Dong and MW Majesky, Arterioscler Thromb Vasc Biol 32 548-551 (2012)
- P Zhang et al, Arterioscler Thromb Vasc Biol 32 756-759 (2012)
- G Gabriely, T Wurdinger, S Kesari, CC Esau, J Burchard, PS Linsley and AM Krichevsky, Molec Cell Biol 28 5369-5380 (2008)
- J Ren, Y Sun, X Zhao, X Wang, J Feng, M Liu and D Zhu, Head Neck Oncol 9 65 (2012)
- A Khanna, S Muthusamy, R Liang, H Sarojini and E Wang, Aging 3 223-236 (2011)
- M Mancini, G Saintigny, C Mahe, M Annichiarico-Petruzzelli, G Melino and E Candi, Aging 4 843-853 (2012)
- Y Oishi, ZW Fu, Y Ohmuki, H Kato and T Noguchi, Br J Dermatol 147 859-868 (2002)