For the complete article, click through to the Nov./Dec. 2019 digital magazine.
Editor's note: The present series serves as a primer for the cosmetic development process. This fourth and final installment covers claims, labeling, manufacturing and market launch. (See: Part I, Part II and Part III.)
Disclaimer: Check with your regulatory specialist to review your specific product and situation.
The development of a new cosmetic product is an extensive, multistep process, from idea generation to market launch. Idea generation brings a general concept for a product to a certain population. After this inspired time, the creation of a new cosmetic will proceed into two main steps: 1) product definition and formulation development and 2) manufacturing, packaging and labeling, before (finally) moving on to market launch. The present article explores the second half of this process.
Efficacy Claims Fundamentals
Product efficacy and related claims are critical elements in the marketing of a cosmetic product, as they are essential tools to inform consumers about a product’s characteristics and quality. They help consumers to choose a product that best suits their needs and expectations.
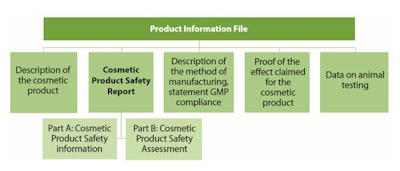
Although in the EU and U.S., as in many countries, there is no regulated list of acceptable or unacceptable claims, claims must be substantiated, truthful and not misleading. For example, in the EU, (EC) 1223/2009 Art. 11.2 (d) indicates that the Product Information File shall contain, “where justified by the nature or the effect of the cosmetic product, proof of the effect claimed for the cosmetic product,” (see Table 1). Furthermore, studies to substantiate claims should be carried out using the final formulation, ideally in finished commercial packaging.
Cosmetics vs. Drugs
The first step to making a product claim is to confirm the new product is, indeed, a cosmetic per regulatory definitions. A cosmetic product is defined in the U.S. and EU as follows:
- U.S. FD&C Act—Section 201 (i): The term ‘‘cosmetic’’ means (1) articles intended to be rubbed, poured, sprinkled, or sprayed on, introduced into, or other-wise applied to the human body or any part thereof for cleansing, beautifying, promoting attractiveness, or altering the appearance, and (2) articles intended for use as a component of any such articles; except that such term shall not include soap.
Among the products included in this definition are: skin moisturizers, perfumes, lipsticks, fingernail polishes, eye and facial makeup preparations, cleansing shampoos, permanent waves, hair colors and deodorants. Section 201(i)(2) excludes soap from the definition of a cosmetic. Also, in the U.S., sunscreens are over-the-counter (OTC), non-prescription drugs.
- EU (EC) 1223/2009—Art. 2: “Cosmetic product” means any substance or mixture intended to be placed in contact with the external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance, protecting them, keeping them in good condition or correcting body odors.
In the EU, soaps, sunscreens and antibacterial shampoos are considered cosmetic products.
U.S. determining factors: In the U.S., if a product is marketed with claims that indicate an intent, for example, to treat or prevent disease, or otherwise affect the structure or function of the human body, including the skin, it is considered a drug. Claims indicating a cosmetic intent deem the product to be a cosmetic. Following are some examples:
- Cosmetic claims: Cleansing, moisturizing, smoothing, freshening, “helps to …,” “improves the appearance of …”
- Drug claims: Treats redness, anti-inflammatory, anti-acne, eczema, psoriasis, anti-septic/anti-bacterial/antimicrobial.
The U.S. Food and Drug Administration (FDA) has issued warning letters to some firms citing drug claims associated with topical skin care, hair care and eyelash/eyebrow preparations noted on both product labeling and websites. Examples of the drug claims cited are acne treatment, cellulite reduction, stretch mark reduction, wrinkle removal, dandruff treatment, hair restoration and eyelash growth.1 This is due, in part, to the FDA’s evaluating cosmetic claims in total context of all wording and images present in labels and collateral promotional literature—including print advertising and websites.
It is also important to note that in the U.S., some products meet the definitions of both cosmetics and drugs. This may happen when a product has two intended uses. For instance, a shampoo is a cosmetic because it is intended to cleanse the hair. An antidandruff treatment is a drug because it is intended to treat dandruff. Consequently, an antidandruff shampoo is both a cosmetic and a drug. Other examples include: antimicrobial cleansers, anti-cavities toothpaste, antiperspirant deodorants, and products marketed with a sun protection claim. All products claiming to provide broad-spectrum SPF protection are regulated as drugs. This also applies to cosmetics and moisturizers labeled with SPF values.
European determining factors: In Europe, various guidance documents have been developed on the delimitation between both cosmetics and drugs,2 and between cosmetics and borderline products, i.e., biocides, medical devices, etc.3 A product is considered a drug either by virtue of its presentation—having properties for treating or preventing disease in human beings—or its function, e.g., restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action.
A list of criteria to be taken into consideration may entail the following aspects: all claims, explicit and implicit, made for the product; the context in which the claims are made; the labeling and packaging; the promotional literature and advertisements, etc.
If the product meets the definition of a drug, it must comply with drug requirements, even if it is also a cosmetic. It is the primary responsibility of the manufacturers/responsible persons to determine the proper classification of the product, as well as insuring that the product complies with all applicable legal requirements.
Requirements for Cosmetic Claims
In the U.S., the FD&C Act—Section 301 prohibits the marketing of adulterated or misbranded cosmetics. “Misbranding” refers to violations involving an improperly labeled or a deceptively packaged product. A cosmetic is misbranded if its labeling is false or misleading in any way.
Continue reading in the Nov./Dec. 2019 digital magazine...