Recently, some European Union member states have expressed concern over the misuse of the Estimated Symbol (℮), often referred to as the “e” mark, on product labels. In addition, some regulators have argued that the International System of Units, known as the metric system, should be used on all product labels to indicate the net contents of a finished product. Both of these concerns have fueled the present column in which the author debates how product labels should indicate the net contents of a cosmetic product. In closing, he comments on the jurisdiction of the CPSC in the United States.
Net Content Regulations in the United States
In the United States, the net contents of a product are regulated by the US Food and Drug Administration (FDA) and the Fair Packaging and Labeling Act (FPLA), which was passed by Congress in 1967. Under 21CFR701.13, the FDA requires net contents to appear on the lower 30% of the principal display panel (PDP), which is what consumers see first, or the outer packaging. The regulation states:
This shall be expressed in terms of weight, measure, numerical count, or a combination of numerical count and weight or measure. The statement shall be in terms of fluid measure if the cosmetic is liquid or in terms of weight if the cosmetic is solid, semisolid, or viscous, or a mixture of solid and liquid. If there is a firmly established, general consumer usage and trade custom of declaring the net quantity of a cosmetic by numerical count, linear measure, or measure of area, such respective term may be used. If there is a firmly established, general consumer usage and trade custom of declaring the contents of a liquid cosmetic by weight, or a solid, semisolid, or viscous cosmetic by fluid measure, it may be used.1
Some may wonder how to interpret this FDA regulation. This author notes that if a product manufacturer fills the product by weight, then it should label that product by weight. Similarly, if the manufacturer fills the product by volume, that product should be labeled by volume.
The regulation also notes: Statements of weight shall be in terms of avoirdupois pound and ounce. Statements of fluid measure shall be in terms of the US gallon of 231 cubic inches and quart, pint, and fluid-ounce subdivisions thereof and shall express the volume at 68°F (20°C).2
The FDA further states: A separate statement of the net quantity of contents in terms of the metric system is not regarded as a supplemental statement and an accurate statement of the net quantity of contents in terms of the metric system of weight or measure may also appear on the principal display panel or on other panels.3
For OTC drugs, the content of each dose must be listed in metric units next to the drug name in the Drug Facts box.4 If the drug has no dose restrictions—for example, topically applied sunscreens, dandruff shampoos, etc.—then the percentage present in the formulation should be used. The FPLA requires net contents be listed on both the PDP and the actual bottle or container.5
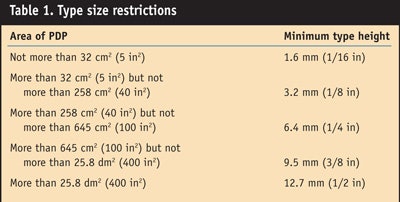
Besides weight metric requirements, there are font size requirements. The type size is restricted to being no less than 1/16 in when the PDP has an area of 5 in2 or less. In the case of PDPs between 5 in2 and 25 in2, the type size cannot be less than 1/8 in, and if PDPs are between 25 in2 and 100 in2, the type size cannot be less than 3/16 in. For PDPs larger than 100 in2, the type size must be at least 1/4 in and for PDPs larger than 400 in2, at least 1/2 in.6 Table 1 shows these restrictions in metric terms.
Net Quantities in Canada
Unlike US regulations, Canada’s Food and Drugs Act regulates the ingredient declarations of a cosmetic product rather than the net quantity of ingredients present by weight or volume. The net quantity of ingredients is instead regulated by the Consumer Packaging and Labeling Act, a statute enforced by the Competition Bureau of Canada.
Certain provinces in Canada such as Quebec require product labels to be printed in both French and English, including net quantity. While a suitable metric symbol is considered bilingual and may be listed as the unit of measure, the use of complete words typically calls for a translation—however, if the correct bilingual abbreviations are used, translation is not required. The correct bilingual abbreviations are listed in Table 2.
Canada mandates that a product be labeled by volume when it is a liquid, gas or viscous substance. When the product is a solid, it should be labeled by weight. Further, a non-metric declaration of net quantity may be provided; however, this information is considered supplementary to the main declaration and it must not be false or misleading to the consumer.
For example, supplementary declarations using American gallons, which are smaller than Canadian gallons, could be misleading to the consumer. In addition, US fluid ounces, which are slightly larger than Canadian fluid ounces, could also be misleading. The label therefore must first list the main declaration in metric units and may then be followed by the supplemental, non-metric units with an indication that the amount is expressed in American gallons, e.g.: 3.79 L (1 gallon US) or 591 mL (20 fl. oz. US).
It should be noted that Canada requires a space between the number and abbreviation, making “100mL” incorrect and “100 mL” correct. Expressions such as “net,” “net weight,” “net contents,” or “net quantity” are not necessary.
Net Contents and the ‘E’ Mark in the EU
Article 6 of the Cosmetic Directive indicates: The nominal content at the time of packaging, given by weight or by volume, except in the case of packaging containing less than five grams or five milliliters, free samples, and single-application packs; for prepackages normally sold as a number of items, for which details of weight or volume are not significant, the content need not be given provided the number of items appears on the packaging.
All net contents listed on EU product labels must use the metric system, but the EU has allowed dual labeling using both the metric and American systems, so long as the metric measurement is listed first. While this practice was to expire in 2009, on June 26, 2007, the EU Commission recommended that dual units be allowed indefinitely. On March 11, 2009, dual labeling was extended to 2019.
In addition to standard units of measure, another symbol can be found on the net contents line on European product labels. While some refer to this symbol as the “e” mark, it is not an “e”—the correct name is the Estimated Symbol, which is shown in Figure 1. Some companies have encountered trouble by displaying this mark incorrectly, which should not occur since this symbol is the Estimated Symbol in 212E from Unicode, as listed by Microsoft Word.
This symbol is voluntary and manufacturers are not required to use it on their product labels. The symbol indicates to the EU that the manufacturer will comply with the EU Regulations of average fill. Thus, if a manufacturer lists it on a product label, they must comply. This symbol only applies to goods shipped within the EU and has no meaning for those shipped into the EU from non-member states.
While some US manufacturers list this symbol on product labels, they typically do so to copy other labels or give the impression that their product is approved in the EU. The United States is a minimum fill country and not an average fill market like the EU. This difference makes it difficult to comply with both market regulations.
Rules for using the Estimated Symbol in the EU include: the average quantity of product in a batch of prepackages shall not be less than the nominal quantity stated on the label; the proportion of prepackages having a negative error greater than the tolerable negative error shall be sufficiently small for batches of prepackages to satisfy the requirements of the official reference test, as specified in legislation; and no prepackage having a negative error greater than twice the tolerable negative error may bear the Estimated Symbol.
Failure to comply with the above requirements, or violations such as incorrect size, location or font, can result in penalties such as the recall of all goods, fines, and even prohibition from conducting business in the EU. Finished products of 5 g or 5 mL or less are not eligible to use this symbol.
Finally, this symbol must be printed ≥ 3 mm on a product label, which may dominate small packages and be visually unappealing.
Metric or Non-metric?
The International System of Units (metric system) is sometimes abbreviated SI for the French “le Système International d’Unités.” Three countries currently do not use the metric system: the United States, Liberia and Myanmar. The United States uses the US customary system for measurement, also known as the American system and sometimes called “English units.” In 1790, Thomas Jefferson proposed a decimal-based measurement system for the United States and in 1792, this decimal-based system was adopted for US currency.7 The Metric Act of 1866 (Public Law 39-183) made it unlawful to refuse trade or deal in metric units. Although the FPLA required English units, it repealed many other laws; however, 39-183 was not one of those repealed.
The big question then becomes: How difficult would it be for the United States to convert all net content declarations in cosmetics to a metric system? Some suggest the American public would not understand and utilize the metric system when purchasing cosmetics. This author believes this is simply not true since every day, millions of Americans buy soda, wine and spirits by the metric system. The nutrition labels on foods are also metric, and American consumers seem to have no problem with that.
In this author’s opinion, the United States should join the rest of the world and use the metric system for personal care products; currently, the United States Metric Association is lobbying for a nationwide conversion to metric. The United States should define net contents by how they are filled and not their form. And in relation to the Estimated Symbol, perhaps if the United States allowed only metric units, the EU would allow the mark to be listed, along with a minimum fill.
Comments on the CPSC
Aside from unrest regarding net content labeling and debate over the metric system, inquiries recently have risen regarding the regulatory jurisdiction of the Consumer Product Safety Commission (CPSC), which was established by the US Congress in 1972 and began functioning in 1973. On Aug. 14, 2008, the Consumer Product Safety Improvement Act of 2008 (Public Law 110–314) (CPSIA) was passed, restricting lead content to 300 ppm in 2009 and 100 ppm by 2011, and banning diethylhexyl phthalate, dibutyl phthalate and benzyl butyl phthalate from toys for children younger than 12 years of age. Major retailers promptly began sending letters to cosmetic companies demanding compliance with this new law. However, personal care products and cosmetics are regulated by the FDA, not the CPSC.
A visit to the CPSC Web site8 will clear up any confusion. Here, the agency states: We have jurisdiction over more than 15,000 kinds of consumer products used in and around the home, in sports, recreation and schools. But we don’t have jurisdiction over some categories of products. They include automobiles and other on-road vehicles, tires, boats, alcohol, tobacco, firearms, food, drugs, cosmetics, pesticides, and medical devices. Our Web site has links to the sites of the federal agencies that do. Therefore, personal care product manufacturers should respond to retailers that this law does not apply to cosmetics unless that cosmetic is represented and sold as a toy. Hopefully, this explanation will clarify the confusion.
Reproduction of this article without permission is strictly prohibited.
References
Send e-mail to [email protected].
1. 21CFR701.13(a)
2. 21CFR701.13(b)
3. 21CFR701.13(r)
4. 21CFR201.66 (c)(2)
5. 15 U.S.C. §§ 1451-1461
6. 21CFR701.13(i)
7. US Metric Association, http://lamar.colostate.edu/~hillger/dates.htm (Accessed Oct 12, 2009)
8. www.cspc.org (Accessed Oct 12, 2009)