The modern concept of wellness is viewed as a balance between respect for the body/skin’s equilibrium and environmental responsibility. Thus, the success of natural-origin ingredients relies upon two key aspects that are linked in a cause-effect relationship. First is the consumer demand for natural and organic products that are safe, do not cause damage to the environment and provide the benefits of natural ingredients. Second is the development of formulas that are as compatible as possible with the physiology of skin and its annexes, without causing any adverse effects or exhibiting allergic potential.1 Although consumers are positively influenced by the growing environmentally friendly lifestyle, they will not accept substandard performances from new products compared to those with which they are familiar. Therefore, formulators are challenged to improve the technologies and strategies of their formulations using only what nature has to offer.
In the search for ingredients such as vegetable oils, waxes, actives, functional ingredients and conditioning agents that can be marketed as environmentally friendly, some categories such as emulsifiers, surfactants and fragrances are forgotten due to the intrinsic difficulty of combining the required high-tech efficacy/functionality with advanced environmental protection.
These are, however, essential in the formulation process in order to guarantee shelf life and to give, preserve and emphasize the sensorial experiences that a cosmetic product must perform. Frequently, the replacement of these ingredients is not easy and the number of emulsifiers that are Ecocert-certified or suitable for an eco-label certification is limited. Thus, the formulator is forced to overcome many difficulties in order to achieve a compromise in terms of stability; in many cases, however, the desired sensorial performance is not reached.
In relation, the aim of the present study was to develop organic, natural and standard formulations using two new emulsifier ingredients of vegetal origin that are Ecocert-certified and that exhibit sensory profile performances equal to formulas made using traditional emulsifiers. This study is focused on the development of these ingredients, using them to optimize the stability of formulations and evaluating the sensorial performances of the final formulas.2
Starting Materials
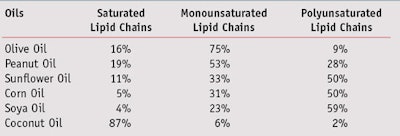
Both emulsifiers developed were based on the chemical combination of hydrophilic protein or amino acids with lipid chains derived from olive oil, which contain lipophilic molecules with known benefits. Olive oil is widely preferred in Europe as a food ingredient3 to other vegetable oils since it exhibits well-known compatibility with body physiology. It is also preferred for its high monounsaturated fatty acid content, which assures better stability to the total lipid complex.4, 5 This stability and functionality is also assured by the relatively high content of unsaponifiable fractions, since the complex is rich in polyphenols and tocopherols. Further, the antioxidant power of olive oil as well as the emollient effects of its lipid moiety, which is kept unchanged in the finished materials, contribute to skin normalization and protection.6 Considering these benefits, extra-virgin olive oil was chosen and obtained by cold pressing the pulp of the fruits of Olea europaea (olive), a species of a small tree of the family Oleaceae native to the coastal areas of the eastern Mediterranean region, which is pictured in Figure 1. Table 1 shows the average composition of olive oil, compared with the most commonly used edible oils.
In the first emulsifier, the lipoprotein potassium olivoyl hydrolyzed oat protein (POHOPa) was incorporated. This material is derived from hydrolyzed oat protein and has a hydrophilic head (see Figure 2). It exhibits mild surface activities, as previously demonstrated by means of a repeated arm wash test. A 10% POHOP solution showed the same behavior as a repeated wash test using only tap water (data not shown). Furthermore, as shown in Figure 3, the addition of POHOP to sodium laureth sulfate (SLES) can reduce its tendency to increase transepidermal water loss (TEWL).
As seen in Figure 3, increaseing POHOP from 0.5% to 2.5% did not decrease the TEWL%. This is the result of a clinical evaluation, and a possible explanation could be that the negative influence of SLES is still predominant over the mild properties of the correcting surfactant.
The second emulsifier links sodium olivoyl glutamate (SOG)b, a vegetal-derived glutamic acid, was linked to the olive oil fatty chains to obtain a hydrophilic head (see Figure 4). This amino acid was selected for its high hydrophilic behavior, so to counterbalance the effect of the lipophile olive oil chain and for its versatile structure with two carboxyl groups, is modified following the pH changes in solution.
In addition to these starting materials, co-emulsifiers such as cetearyl alcohol, glyceryl stearate and glyceryl oleate were added to the POHOP and SOG blends to produce the final emulsifiers:7 (25–50% POHOP; 25–50% cetearyl alcohol; 25–50% glyceryl stearate; and 5–10% glyceryl oleate) and (25–50% SOG; 25–50% cetearyl alcohol; and 25–50% glyceryl stearate).
These emulsifier blends are prepared in order to balance the polarity of the main molecule, POHOP and SOG, and to create the emulsifying properties. As for their environmental impact, the two ingredients are characterized by high biodegradability, according to the CEE regulation N.82/242 OECD Method.
Methods
Evaluation of the emulsifying properties of both ingredients was carried out using a systematic approach, conducting different trials with different ingredients, and added one at a time and at different amounts. Simple emulsion formulas containing one of the two emulsifier systems and a combination of three different oils, i.e., hydrocarbons, esters and triglycerides, were assessed. These model formulas could serve as a basis for developing several finished products, as will be discussed.
Different emulsions were prepared by changing various parameters, including: the polarity of the oil phase, to identify the polarity of the emulsifiers; the order of addition of the ingredients, to improve the stability of and refine the emulsions; the addition of stabilizing polymers, to evaluate their effects and compatibility with the emulsifiers; the amount of oil phase, to identify limits of the ingredients’ emulsifying properties; and the percentage of the emulsifiers, to investigate their behavior under extreme conditions, e.g., in systems with high amounts of oil phase. The results obtained for each of the two emulsifiers, reported later in this article, are shown separately for clarity.
Each emulsion was checked under standard and accelerated stability conditions for homogeneity and particle size by a microscopec and centrifuged for one month at 4°C, 43°C and RT. Figure 5 shows the three main variations in appearance of the emulsions, which were used for comparison purposes to evaluate all formula samples for particle size and homogeneity. If the emulsion appeared similar to Figure 5A, it was considered not fine and not homogeneous; if similar to Figure 5B, it was evaluated as quite fine and homogeneous. When the emulsion looked like Figure 5C, it was considered as very fine and homogeneous.
Samples were also visually assessed for accelerated stability and therefore stored in glass jars for 30 min at 35°C and 7000 Gravity before being evaluated. Figure 6 shows the three main variations in appearance of the emulsions, which were used for comparison purposes to assess all formula samples for stability. The emulsion was considered stable if it appeared as in Figure 6A, separated as in Figure 6B, or not physically stable as in Figure 6C. If separation was lower than 10%, emulsions were evaluated as quite stable.
Finally, sensory assessments were made by a panel of trained experts8, 9 who evaluated all formulations prepared with the different oils and different polarities, including two final formulations—an eye contour cream (see Formula 1) and a lenitive face cream (see Formula 2). In addition, consumer assessments were carried out.
POHOP Emulsifier
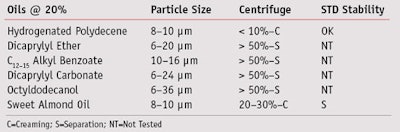
Oil polarity: To evaluate the effects of oil phase polarity on the POHOP emulsion, oils having increasing polarity, shown in Table 2, were used to prepare emulsions with POHOP (5%). It can be seen that the best performances were obtained with nonpolar oils such as hydrogenated polydecene, and partially with polar oils such as sweet almond oil. POHOP yielded a satisfactory particle size and good stability when centrifuged with both oils. The standard stability test yielded good results only with hydrogenated polydecene.
Production method: The next step was to evaluate the order of addition of the various emulsion components, as shown in Figure 7. With method 1, the emulsifier was first added to the oil and, at the right temperature, the oil phase was added to the water phase. With method 2, the emulsifier was first added to the oil, and then a little amount of the water phase was added to the oil phase for the emulsion process. The remaining part of the water phase was used to cool the system. Finally, in method 3, the emulsifier was added to the water phase.10 Of these three approaches, method 2 was shown to be the best because the resulting emulsions exhibited a particle size of 2–6 mm, which was five to six times smaller than the size obtained with the other methods. Moreover, the accelerated and standard stability tests were satisfactory for the three oils employed: nonpolar hydrogenated polydecene, medium polar C12–15 alkyl benzoate and polar sweet almond oil.
Stabilizing polymers: Three different polymers were used in the emulsions to test their compatibility of the POHOP emulsifiers, and to improve the quality of the emulsions. The polymers used included xanthan gum (0.15%), hydroxyethylcellulose (0.4%) and carbomer (0.4%). The emulsions were prepared using the three previously mentioned oils at 20% with POHOP (5%) and via production method 2.
All polymers tested showed good results with the POHOP emulsifier. In particular, the particle size of the xanthan gum emulsion was ≤ 2 mm with all oils, and all the emulsions were found to be stable. The sequence in which the polymers were added was also important. In the case of xanthan gum, it was essential to add the polymer before the emulsification process, which improved the fineness of the emulsion. And while no significant difference was found with hydroxyethylcellulose, carbomer had to be added after the emulsification process because ions in the emulsifier could interfere with this polymer, which is known to be salt-sensitive.
Change in emulsifier, oil amount: Finally, the emulsifying properties were tested by changing the amounts of the oil phase and emulsifier used. For these studies, sweet almond oil at 10%, 20%, 30% and 50% were used, and POHOP (5%) was used at levels varying from 2.5% and 7.5%, to 10% and 20% with all three oils—i.e., nonpolar hydrogenated polydecene, medium polar C12–15 alkyl benzoate and polar sweet almond oil. Using production method 2 and adding a little amount of xanthan gum, the emulsifier enabled the creation of an emulsion with 50% oil phase. In general, if the percentage of oil phase was within the range of 10–20%, the amount of emulsifier stayed within the 3–4% range. Higher amounts of oil phase required 5–6% emulsifier.
SOG Emulsifier
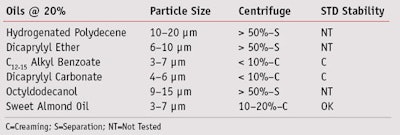
Oil polarity: By applying the same methods, the behavior of SOG with oils of different polarity was considerably different than the behavior of the POHOP emulsifier. Indeed, the best performances were obtained with medium polar oils such as C12–15 alkyl benzoate and dicaprylyl carbonate, and partially with polar oils such as sweet almond oil (see Table 3).
Production method: As in the previous evaluation, the tests carried out with different methods showed the best approach was using method 2. The emulsions produced in this manner exhibited a good particle size; < 2 μm with medium polar oil (C12–15 alkyl benzoate); 2–5 μm with polar oil (sweet almond oil); and 5–10 μm with nonpolar oil (hydrogenated polydecene). The accelerated and standard stability tests also were satisfactory with nonpolar oils.
Stabilizing polymers: The three polymers tested yielded good results; however, the best results were with xanthan gum. The particle size was ≤ 2 μm with the three tested oils, in the same way as previously observed with POHOP, and all the prepared emulsions were stable in the centrifuge and when tested for standard stability. No negative interactions were noted between carbomer and SOG.
Change in emulsifier, oil amount: Finally, emulsions with 35–40% oil phase were achieved by changing the amounts of the oil phase and emulsifier used. For these studies, 10%, 20%, 30% and 50% hydrogenated polydecene were used, and the amounts of SOG (5%) used ranged from 2.5% and 7.5%, to 10% and 20% with all three oils— i.e., C12–15 alkyl benzoate, dicaprylyl carbonate and sweet almond oil. Production method 2 was used, and a small amount of xanthan gum was added. In general, if the percentage of oil phase was within a range 10–20%, the amount of emulsifier required was in the 4–5% range. Higher amounts of oil phase required 5–7.5% emulsifier.
Summary
Both emulsifiers tested were found to be suitable for a wide range of applications. Their emulsification properties were comparable to standard emulsifiers. POHOP was slightly more powerful than SOG because it formed stable, homogeneous and fine emulsions with formulas containing 50% oil phase as required, for instance, in high SPF sunscreens, having a very high amount of internal oil phase at a 6% concentration. Moreover, the emulsification properties between the two were quite different. With POHOP, the best performance was obtained using polar and nonpolar oils while SOG blended better with the oil phase mainly using medium polar oils. However, the addition of stabilizing polymers and the implementation of the production process made the emulsifiers poly-functional toward the oil blends used.
As for POHOP, the order of the production process was especially important when using a salt-sensitive polymer. It was also found that, as happens for standard emulsifiers, higher amounts were needed in the presence of a higher amount of solids, such as inorganic filters. Finally, the complementary characteristics of the two emulsifiers, in terms of polarity in the oil phase, allowed for the stability of the finished formula to be adjusted by optimizing the addition of both emulsifiers (see Formula 3).
Sensory Evaluations
Panel of experts: As noted, each formula produced was evaluated in terms of initial perception, perception during application and final perception by an expert panel. Significant differences were recorded between the two emulsifiers only at the final perception stage. All emulsions with different oils made with POHOP and SOG had a light initial perception, regardless of the oil used. This perception held true even with systems containing high amounts of vegetable oil—i.e., sweet almond oil.
The spreadability during application of the emulsions made with both emulsifiers was related to the oil used, whereas the final perception of the emulsions made with POHOP gave an emollient feel irrespective of the oil. The emulsions made with SOG showed a final perception in accordance with the used oil; the final feel was perceived as nourishing when polar oils were used while, a dry and emollient feel was perceived with medium polar and nonpolar oils.
Consumer panel: Considering the results of the panel of experts, two finished products were prepared and their sensorial profiles evaluated for softness, smoothness and stickiness. The tests were performed using a trained panel of 20 participants whose responses were recorded.
After the application of the product (Formula 1), almost 65% of volunteers perceived their skin as feeling soft and smooth while 40% of them did not perceive any sticky feel. Improved results were achieved with Formula 2; after application, 50% of the volunteers perceived their skin as soft; 65% perceived it as smooth; and almost 80% of the volunteers did not perceive any sticky feel.
Additional Formulations
Additional formulations (see Formulas 3 and 4) were then prepared to test the emulsification properties of the two emulsifiers further and assess their range of application. After the systematic approach in which the properties of the emulsifier are understood, finished formulas can be made without using the same systematic approach but just applying a standard formulation development procedure. This is made also to demonstrate how to use the new ingredients in complete formulas.
In particular, the combination of the two emulsifiers used in Formula 3 was chosen based on the presence of a high amount of oil phase and high levels of medium polar oils. It was found that 7.5% POHOP (5%) guaranteed a higher emulsification power while 2.5% SOG (5%) adjusted the system stability (data not shown).
Conclusion
The results obtained here suggest that POHOP and SOG could serve as valid, vegetal-derived alternatives to common ethoxylated and polymeric emulsifiers with equal performances in terms of emulsification properties and sensorial profiles. These materials did not show any significant incompatibilities with the cosmetic ingredients used, and imparted a light perception to the formulation irrespective of the amount or polarity of the fatty phase, especially in relation to initial perception. The individual components belong to the prepared POHOP or SOG blends (in a solid form). The ability of the emulsifying ingredients inventor was to find the equilibrium percentage among all ingredients for getting the equilibrate combination. The two ingredients could be used alone or in combination, depending on the structure of the final system.
Today’s formulator is continuously faced with the problem of using ingredients like emulsifiers with high performance requirements, and at the same time respect the environment and the need for using renewable sources for cosmetic ingredients. These two new emulsifiers combine performances and environmental respect, while allowing the adoption of a wide range of oils for the internal phase of o/w emulsions.
References
Send e-mail to [email protected].
1. L Rigano, G Giammarrusti and F Rastrelli, Vegetable Oils—The Base of New Active Principles, SÖFW Journal 132 1–2, 25–33 (2006)
2. L Rigano and S Sirigu, Analisi sensoriale: uno strumento della qualità in cosmetica, Cosmesi Dermatologica 47 81–94 (1997)
3. http://r0.unctad.org/infocomm/anglais/olive/market.htm#conso, United Nations Conference on Trade and Development (Accessed Jun 16, 2011)
4. L Sousselier, Novel ingredients from Olive-nature’s gift for beauty, conference presented during PCIA Seoul, March 2001
5. AK Kiritsakis, Virgin olive oil composition and its effect on human health, Inform 13 3 237–241 (2002)
6. R Watts and L Sousselier, Fruits of the gods, Soap, Perfumery & Cosmetics 63–68 (Oct 2000) 7. M D’Angelo, G Proserpio and GB Rastrelli, An Olive Oil- and Wheat-derived Lipoprotein Emulsifier, Cosm & Toil 116 8 45–46 (2001)
8. IFSCC Monograph, Priciple of Product Evaluation: Objective Sensory Methods 1, 7 (1995)
9. H Stone and JL Sidel, Sensory evaluation practices, Academic Press Inc. (1995)
10. TJ Lin, Manufacturing Cosmetic Emulsions: Pragmatic Troubleshooting and Energy Conservation, Allured Publishing Corp., Carol Stream, IL (2010)