Skin is exposed to the external environment that brings with it daily aggressions such as UV light, chemicals, pollution, temperature, etc. These aggressions can create skin irritation, especially in sensitive skin individuals, leading to itching and discomfort. Moreover, in the long-term, irritation leads to skin damage and premature aging as a result of elastosis and matrix degradation.1-3 It is therefore important to stop skin irritation rapidly to not only reduce skin discomfort, but also avoid further skin damage.
Skin irritation is sustained by a cross-talk mechanism between a keratinocyte in the epidermis layer and the infiltrating immune cell, e.g. T lymphocytes. This cross-talk creates an amplification loop that leads to overreaction and escalates the inflammatory process with consequent skin erythema and irritation. Under these circumstances, the skin is unbalanced and requires re-balancing.
NF-κB and JAK/STAT Pathways
The NF-κB and JAK/STAT signaling pathways are both dramatically amplified in dermatological conditions and play substantial roles in sustaining skin irritation.4 In the NF-κB pathway, a cytokine receptor expressed on the cell membrane of the keratinocyte will recognize cytokine ligands produced by skin-infiltrating immune cells, thus triggering the release in the cytoplasm of the NF-κB unit, which is normally sequestered by a family of inhibitory proteins known as inhibitors of NF-κB (IκBs). The signaling pathway from the receptor will lead to the liberation and nuclear accumulation of NF-κB, in turn activating the transcription of pro-inflammatory molecules such as cytokines and chemokines.5, 6
Similarly, in the JAK/STAT pathway, a cytokine receptor expressed on the cell membrane of the keratinocyte will recognize cytokine ligands produced by the skin-infiltrating immune cell. However, this triggers a series of phosphorylation KEY WORDS: soothing, irritation, NF-κB, JAK/STAT, keratinocyte mechanisms leading to the translocation of STAT dimers to the nucleus, also subsequently activating the transcription of pro-inflammatory molecules such as cytokines and chemokines.7 To interrupt this cross-talk and thus re-balance overreacting skin, specific mechanisms involved in this loop can be targeted; the present work describes two bioactive complexes designed to act on the NF-κB and JAK/STAT signaling pathways.
EG Complex and NF-κB Target
To target the NF-κB pathway, a water-soluble complexa was developed based on a blend of gallyl glucoside, epigallocatechin gallatyl glucoside and propyl gallate; in the present article, this blend will be referred to as the EG complex. Gallyl glucoside and epigallocatechin gallatyl glucoside are, respectively, glycosylated forms of: gallic acid extracted from oak and purified, and epigallocatechin gallate extracted from green tea and purified. Glycosylation was carried out by adding a glucose group to the aglicone form by a bacterial enzyme. The resulting glucosides proved to be more stable to degradation than the aglicone forms and much more water-soluble (data not shown).
Extensive work has been published on the anti-inflammatory action of gallic acid. For instance, gallic acid has been shown in vitro to inhibit histamine release and cytokine production in mast cells8 and in monocytes.9 Furthermore, its activity in inhibiting NF-κB pathway has been suggested.10, 11 In the case of epigallocatechin gallate, the immunomodulating properties of green tea are To interrupt cross-talk and thus re-balance overreacting skin, specific mechanisms can be targeted. well-known.12 Epigallocatechin gallate is responsible for several actions against inflammation; specifically, it has been shown to reduce immune cell infiltration in human skin,13 decrease IL-8 release in human keratinocytes,14 and inhibit NF-κB activation in human15 and mouse keratinocytes.16, 17 Finally, propyl gallate has historical evidence as an anti-irritant18 and a lipoxygenase inhibitor.19, 20
PN Complex and JAK/STAT Target
To target the JAK/STAT pathway, a lipid-soluble complexb was formulated based on a combination of panthenyl triacetate (PTA) and naringenin; in the present article, this blend will be referred to as the PN complex.
Panthenyl triacetate is a derivative of panthenol and precursor of panthotenic acid. Extensive literature exists showing panthenol’s ability to act as a skin moisturizer, improve the skin barrier, increase wound healing and reduce UV-induced erythema.21 In relation to anti-irritation properties, panthenol has been used as an adjuvant of hydrocortisone in clinical studies22 or as a possible alternative.23–25 Interestingly its role in modulating the immune system was investigated in 1981 by Axelrod,26 and more recently, panthenol has been shown to modulate gene expression in stress-induced fibroblasts.27
Naringenin is a flavonoid that is particularly abundant in citrus fruits. It has been used as a food supplement for its health benefits, being an antioxidant, a free radical scavenger and a metabolism regulator. Recent evidence has shown naringenin’s ability to repair DNA damage in UV-irradiated keratinocytes28 and since DNA damage is linked to skin inflammation,29, 30 naringenin may have a major role in decreasing inflammation. This hypothesis is sustained by other work that shows the activity of naringenin in inhibiting pro-inflammatory cytokines in fibroblasts.31 In addition, the capacity of naringenin to decrease NF-κB and JAK/STAT signaling pathways was shown in different human tissues in inflammatory states.32, 33
Experimental Approach
The hypothesis was then to test, in human keratinocytes, the ability of the EG and PN complexes to inhibit the NF-κB and JAK/STAT signaling pathways and subsequent cytokine and chemokine expression and release leading to pro-inflammatory cross-talk and skin irritation (see Figure 1). In the experimental setting, keratinocytes were induced to a pro-inflammatory status by incubating them with a different cytokine mixture known to induce the activation of the NF-κB and JAK/STAT pathways, respectively.4, 34
These experiments were then validated in vivo on human volunteers by testing the EG or PN complex at different concentrations and different vehicles as immediate soothing agents after an induced irritation.
In vitro Studies: Signaling, Cytokine and Chemokine Expression
Normal human epidermal keratinocytes (NHEK) were cultured under standard conditions and incubated with either the EG or PN complex and the control references at different concentrations for 24 hr. Tests employing the EG complex were compared with 5 mM NF-κB inhibitor III and 100 mM dexamethasone; for the PN complex, the JAK/STAT inhibitor pyridone 6 served as the control.
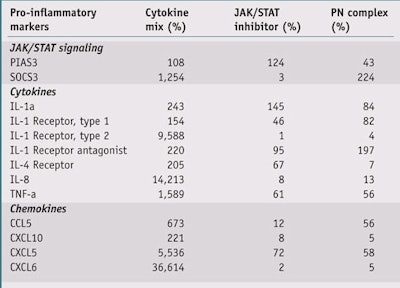
After incubation, cells were induced with a cytokines mix: TNF-a + IL-1a (both at 5 ng/mL) for the EG complex and Oncostatin M + IL-17 + TNF-a at 3 ng/mL for the PN complex. NHEK incubated with the PN complex were extracted for mRNA after 24 hr, the mRNA was reverse-transcribed, and genes for JAK/STAT signalling as well as chemokines and cytokines were analyzed (see Table 1). NHEK incubated with the EG complex were also analyzed after 24 hr for IL-8 and CXCL1 release via the enzyme-linked immunosorbent assay (ELISA). All experimental conditions were performed at n = 3.
To test for NF-κB activation, transformed human cells (HT29) transfected with a NF-κB reporter plasmid linked to alkaline phosphatasec were incubated for 24 hr with the NF-κB cytokine activator TNF-a (20 ng/mL) in the presence or in absence of the EG complex at different concentrations. A colorimetric reaction followed to determine the reporter gene activity.
Results
PN complex: As shown in Table 1 and Figures 2, 3 and 4, treatment of NHEK with the Oncostatin M + IL-17 + TNF-a cytokine mix dramatically increased the transcription of JAK/ STAT signaling markers, cytokines and chemokines mRNA; by 681%, 3,744% and 10,761%, respectively. Treatment with the JAK/STAT inhibitor pyridone 6 (5 mM) significantly inhibited this increase (p < 0.001, t-test), as did treatment with the PN complex (0.4%) at a similar level (p < 0.001, t-test). These findings support the PN complex as a strong inhibitor of the JAK/STAT inflammation pathway.
EG complex: Treatment of HT29 cells with the EG complex at different concentrations dramatically inhibited TNF-a induced activation of an NF-κB reporter plasmid. This inhibition was dose-dependent (see Figure 5) and statistically significant at all concentrations used (z < 1.96, Wilcoxon test), with a max inhibition at 1.1% (-85% vs cytokine mix-induced). Treatment of NHEK with the TNF-a + IL-1a cytokine mix dramatically increased the release of pro-inflammatory interleukine IL-8 and chemokine CXCL1. This increase, in comparison with the untreated control NHEK, was +98.2% for IL-8 and +66.6% for CXCL1 (see Figures 6 and 7).
Treatment with control references NF-κB inhibitor (5 mM) and dexamethasone (100 mM) significantly inhibited the cytokine mix-induced IL-8 and CXCL1 increase (p < 0.01, t-test). The EG complex, at increasing concentrations, also exhibited a strong and significant inhibition activity on IL-8 release; 99% inhibition at 0.4%, compared with the cytokine mixinduced NHEK (p < 0.001, t-test, see Figure 6). In addition, the EG complex was even able to reduce the basal activity of CXCL1 release; 135% inhibition at 0.4%, compared with the cytokine mix-induced NHEK (p < 0.001, t-test, see Figure 7). This data strongly supports the EG complex as an inhibitor of the NF-κB inflammation mediated pathway.
In vivo Studies: Clinical Evaluation of Immediate Skin Soothing
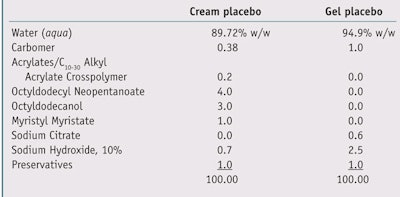
Two different groups of 25 volunteers each consisting of both men and women were used to test the EG and PN complexes. Skin irritation was induced through the application of epicutaneous patches including eight chambersd filled with 2% SLS aqueous solution applied to the backs of the volunteers. The patches were removed 24 hr after application, and as a measure of skin irritation and damage, the erythema index and transepidermal water loss (TEWL) were evaluatede, f 15 min after the removal of patches (T0). After skin irritation was induced, test formulas (see Table 2) were applied blindly following their usual use to volunteers. In the first group of volunteers, four of the irritated areas were treated with a water-based gel containing the EG complex at varying concentrations; in the second group of volunteers, four irritated areas were treated with a cream containing the PN complex at different concentrations. In both groups of volunteers, two irritated areas were treated with a placebo and two were left untreated.
In the case of the EG complex, the test water-based gels contained (or not) the EG complex at concentrations of 1.0% and 3.0%. In the case of the PN complex, the test creams contained (or not) the PN complex at concentrations of 0.5% and 2.0%. Samples were left on the irritated areas for 15-min, 30-min, 60-min and 120-min time intervals. The erythema index and TEWL were evaluated at each time point and comparisons were with all test samples and untreated areas. Data was analyzed and expressed as percentage variations versus T0; statistical significance also was calculated.
Results
EG complex gel: In human volunteers, skin irritation induced for 24 hr by SLS patch occlusion was immediately soothed by the gel containing the EG complex, as measured by a reduced erythema index after only 15 min. The data was significant (p < 0.05, t-test) for the full range of testing with the highest concentration of EG complex (3.0%), compared with a placebo gel. The lowest concentration of EG complex (1.0%) reached significance (p < 0.001, t-test) after 120 min of application (see Figure 8). Also at 120 min of application, the gel containing 3.0% of the EG complex reached an erythema reduction of -15%.
In the same volunteers, SLS-induced TEWL was reduced by the gel containing the EG complex at 3.0% and 1.0%. The data was significant for the gel containing the 3.0% EG complex (p < 0.05, t-test) but not for the 1.0% complex after 30 min, 60 min and 120 min, when compared with a placebo gel . At 120 min of application, the 3.0% EG complex gel reduced TEWL by 23% (see Figure 9). The reduction in TEWL suggests a healing effect on the skin barrier.
PN complex cream: In human volunteers, skin irritation induced for 24 hr by SLS patch occlusion was immediately soothed by the cream containing the PN complex, as measured by a reduced erythema index after only 15 min. The data was significant for the 2.0% PN complex cream (p < 0.05, t-test), when compared with a placebo cream at 30 min, 60 min and 120 min. The effects of the 0.5% PN complex cream were significant after 60 min and 120 min of application (p < 0.01, t-test). Also at 120 min of application, the cream containing the 2.0% PN complex reduced the erythema index by 13% (see Figure 10).
Overall, for both the 2.0% and 0.5% PN test creams, the soothing effect increased with time. This is due to the physiological reaction of the skin to the active as well as its increased bioavailability since PTA must be deacytilated in order to act.
In the same volunteers, SLS-induced TEWL was reduced by the cream containing the PN complex at 2.0% and 0.5%. The data was significant for the cream containing 2.0% of the PN complex (p < 0.05, t- test) after 15 min, 30 min, 60 min and 120 min, when compared with a placebo cream. At 120 min of application, the cream containing the 2.0% complex reduced TEWL by 15% (see Figure 11). The reduction in TEWL suggests a healing effect on the skin barrier.
Conclusions
The present article demonstrates how test complexes EG and PN inhibit the transcription and subsequent release of pro-inflammatory cytokines and chemokines in human keratynocytes induced to an inflammatory status. This inhibition was associated to JAK/ STAT and NF-κB targeting (see Figure 2 on Page 33 and Figure 5 on Page 34). The mRNA transcription of a series of cytokines and chemokines (described in Table 1) was reduced, as well as the release of pro-inflammatory molecules such as interleukine IL-8 and chemokine CXCL1 (see Figures 6 on Page 34 and Figure 7 on Page 35).
When the EG and PN complexes were incorporated in cosmetic vehicles at different concentrations and applied to the irritated skin of human volunteers, the formulas soothed the irritation in as soon as 15 min, and both the lowest and the highest concentrations were effective in a dose-dependent manner. Interestingly, the EG complex was rapidly effective and reached a plateau at 120 min of application, while the PN complex built in efficacy over a longer period of time. This could be explained with the longer time required for the PTA contained in the PN complex to deacetylate to its active form, panthotenic acid, in the skin (data not shown). Both complexes EG and PN also were able to decrease TEWL induced by the irritation treatment; this dose-dependent effect was statistically significant after 15 min of application, increasing over time.
In conclusion, the authors have demonstrated by in vitro and in vivo studies that the EG and PN complexes are indeed strong anti-irritation ingredients. Moreover, their effect in reducing TEWL suggests a role as healing agents to restore damaged skin barrier.
References
Send e-mail to [email protected].
1. MF Bennett, MK Robinson, ED Baron and KD Cooper, Skin immune systems and inflammation: Protector of the skin or promoter of aging? J Invest Dermatol Symp Proc 13 15–19 (2008)
2. CR Thornfeldt, Chronic inflammation is etiology of extrinsic aging, J Cosmet Dermatol 7 78–82 (2008)
3. PU Giacomoni and G Rein, A mechanistic model for the aging of human skin, Micron 35 179–184, (2004)
4. FX Bernard et al, Cell signaling in psoriasis for ingredient evaluation and product design, Cosmet & Toil 124 42–48 (2009)
5. F Wan and MJ Lenardo, The nuclear signaling of NF-kappaB: Current knowledge, new insights and future perspectives, Cell Res 20 24–33 (2010)
6. M Pasparakis, Regulation of tissue homeostasis by NF-kappaB signalling: Implications for inflammatory diseases, Nat Rev Immunol 9 778–788 (2009)
7. DS Aaronson and CM Horvath, A road map for those who don’t know JAK-STAT, Science 296 1653–1655 (2002)
8. SH Kim et al, Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells, Toxicol Sci 91 123–131 (2006)
9. G Kuppan, J Balasubramanyam, F Monickaraj, G Srinivasan, V Mohan, M Balasubramanyam, Transcriptional regulation of cytokines and oxidative stress by gallic acid in human THP-1 monocytes, Cytokine 49 229–234 (2010)
10. KC Choi et al, Gallic acid suppresses lipopolysaccharide- induced nuclear factor-kappaB signaling by preventing RelA acetylation in A549 lung cancer cells, Mol Cancer Res 7 2011–2021 (2009)
11. KW Lee, JK Kundu, SO Kim, KS Chun, HJ Lee and YJ Surh, Cocoa polyphenols inhibit phorbol ester-induced superoxide anion formation in cultured HL-60 cells and expression of cyclooxygenase-2 activation of NF-kappaB and MAPKS in mouse skin in vivo, J Nutr 136 1150–1155 (2006)
12. SK Katiyar, Skin photoprotection by green tea: Antioxidant and immunomodulatory effects, Curr Drug Targets Immune Endocr Metabol Disord 3 234–242 (2003)
13. SK Katiyar, MS Matsui, CA Elmets and H Mukhtar, Polyphenolic antioxidant (-)-epigallocatechin- 3-gallate from green tea reduces UVB-induced inflammatory responses and infi ltration of leukocytes in human skin, Photochem Photobiol 69 148–153 (1999)
14. S Trompezinski, A Denis, D Schmitt and J Viac, Comparative effects of polyphenols from green tea (EGCG) and soybean (genistein) on VEGF and IL-8 release from normal human keratinocytes stimulated with the proinfl ammatory cytokine TNFalpha, Arch Dermatol Res 295 112–116 (2003)
15. F Afaq, VM Adhami, N Ahmad and H Mukhtar, Inhibition of ultraviolet B-mediated activation of nuclear factor kappaB in normal human epidermal keratinocytes by green tea constituent (-)-epigallocatechin-3-gallate, Oncogene 22 1035–1044 (2003)
16. JK Kundu and YJ Surh, Epigallocatechin gallate inhibits phorbol ester-induced activation of NF-kappa B and CREB in mouse skin: Role of p38 MAPK, Ann N Y Acad Sci 1095 504–512 (2007)
17. F Afaq, N Ahmad and H Mukhtar, Suppression of UVB-induced phosphorylation of mitogenactivated protein kinases and nuclear factor kappa B by green tea polyphenol in SKH-1 hairless mice, Oncogene 22 9254–9264 (2003)
18. JS Franzone, T Natale, R Cirillo and MV Torrielli, Infl uence of propyl-gallate and 2-mercaptopropionylglycine on the development of acute inflammatory reactions and on biosynthesis of PGE2, Boll Soc Ital Biol Sper 56 2539–2545 (1980)
19. C Kuhn, H Bekemeier, R Hirschelmann and AJ Giessler, Action of cyclooxygenase (COX) and lipoxygenase (LOX) inhibitors as well as of oxygen free radical scavengers (OFRS) in the infl ammation-induced vasodepression, Biomed Biochim Acta 47 S320–S323 (1988)
20. B Bosman, Testing of lipoxygenase inhibitors, cyclooxygenase inhibitors, drugs with immunomodulating properties and some reference antipsoriatic drugs in the modifi ed mouse tail test, an animal model of psoriasis, Skin Pharmacol 7 324–334 (1994)
21. F Ebner, A Heller, F Rippke and I Tausch, Topical use of dexpanthenol in skin disorders, Am J Clin Dermatol 3 427–433 (2002)
22. PR Kline, Topical treatment of itching dermatoses; Use of a combination of hydrocortisone and panthenol, Med Times 84 148–157 (1956)
23. LF Eichenfi eld, JF Fowler Jr, DS Rigel and SC Taylor, Natural advances in eczema care, Cutis 80 2–16 (2007)
24. K Biro, D Thaçi, FR Ochsendorf, R Kaufmann and WH Boehncke, Efficacy of dexpanthenol in skin protection against irritation: A doubleblind, placebo-controlled study, Contact Dermatitis 49 80–84 (2003)
25. E Proksch and HP Nissen, Dexpanthenol enhances skin barrier repair and reduces inflammation after sodium lauryl sulphateinduced irritation, J Dermatolog Treat 13 173–178 (2002) 26. AE Axelrod, Role of the B vitamins in the immune response, Adv Exp Med Biol 135 93–106 (1981) 27. T Wiederholt et al, Calcium pantothenate modulates gene expression in proliferating human dermal fi broblasts, Exp Dermatol 18 969–978 (2009)
28. MA El-Mahdy et al, Naringenin protects HaCaT human keratinocytes against UVBinduced apoptosis and enhances the removal of cyclobutane pyrimidine dimers from the genome, Photochem Photobiol 84 307–316 (2008)
29. DB Yarosh, DNA repair, immunosuppression and skin cancer, Cutis 74 10–13 (2004)
30. C Petit-Frère et al, Induction of interleukin-6 production by ultraviolet radiation in normal human epidermal keratinocytes and in a human keratinocyte cell line is mediated by DNA damage, J Invest Dermatol 111 354–359 (1998)
31. C Bodet, VD La, F Epifano and D Grenier, Naringenin has anti-infl ammatory properties in macrophage and ex vivo human whole-blood models, J Periodontal Res 43 400–407 (2008)
32. Y Shi et al, Naringenin inhibits allergen-induced airway inflammation and airway responsiveness and inhibits NF-kappaB activity in a murine model of asthma, Can J Physiol Pharmacol 87 729–735 (2009)
33. K Vafeiadou, D Vauzour, HY Lee, A Rodriguez- Mateos, RJ Williams and JP Spencer, The citrus fl avanone naringenin inhibits infl ammatory signalling in glial cells and protects against neuroinflammatory injury, Arch Biochem Biophys 484 100–109 (2009)
34. S Vallabhapurapu and M Karin, Regulation and function of NF-kappaB transcription factors in the immune system, Annu Rev Immunol 27 693–733 (2009)