
[Update] Editor's note: Several very astute readers have called to our attention an error in the previous version of Figure 1 for this article, wherein the "a" and "b" units were reversed. The below has been updated with the correct configuration. We hope this clarifies any confusion.
The most important factor that determines if a PEG-8 dimethicone polymer is soluble, insoluble or dispersible in water is the ratio of D:D* units present in the molecule.
Note: D* refers to water soluble groups; D refers to silicone soluble groups.
This critical parameter is not reflected in the INCI name. The D:D* ratio is determined as follows: the number of D units (i.e., the number of dimethyl containing groups, or a) divided by the number of D* units (i.e., the number of organofunctional units, or b). See Figure 1.
Figure 1. PEG-8 dimethicone structure (generic)
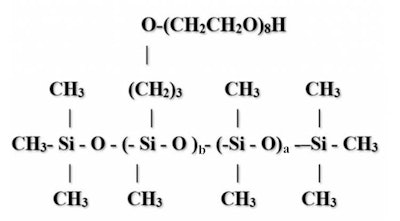 If the result is less than or equal to 3, the silicone compound will be water soluble. If the number is 4 or higher, the polymer will not be soluble.
If the result is less than or equal to 3, the silicone compound will be water soluble. If the number is 4 or higher, the polymer will not be soluble.The ability to regulate the structure of a silicone polymeric PEG/PPG surfactant to form a microemulsion in a formulation presents a useful mechanism to maximize deposition. Simply put, if PEG/PPG dimethicone is too water soluble, it will wash down the drain during rinsing and provide little or no additional benefit to the formulation. Thus, in order for PEG/PPG dimethicone to remain clear and soluble and efficiently impart lasting deposition in a system, the PEG/PPG dimethicone used should form a microemulsion in the formula tested.
The simplest way to achieve this is to alter the D/D* ratio (the ratio of a:b). In doing so, the solubility is altered such that the least soluble product without insolubility can be added to a system, in turn depositing to the substrate with the lowest free energy. Put another way: if you want the best delivery on a substrate from a clear formulation, deliver the silicone polymer as a microemulsion.
Figure 2 shows the effect of altering the D/D* ratio in PEG dimethicone. In fact, it is relatively easy to use a calculation to effectively determine the number of PEG-8 units in a silicone polymer to make a microemulsion.
Figure 2. PEG-8 dimethicone solubility
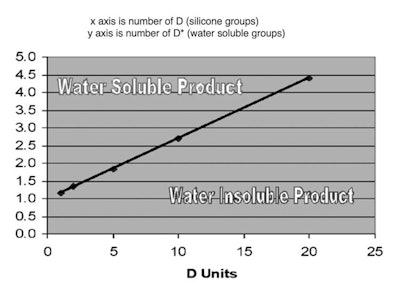
The calculation to determine PEG-8 dimethicone solubility is as follows:
Minimum Needed b Units = (0.1499)(Actual Number of a Units) + 1
where a and b are the subscripts shown in Figure 1. This means if the number of b units is above the calculated value, an appreciable concentration of insoluble oligomer would not be present. If the value of b in the polymer is lower than that required in the calculation (based on the number of a subunits), a soluble product is impossible since there will be an appreciable amount of insoluble polymer present in the oligomer mixture.
Choosing a D:D* ratio to provide a silicone polymer that forms a microemulsion is a simple, cost-effective method to improve the efficiency of a PEG/PPG dimethicone in formulation. And remember, the INCI name for all ratios of D:D* is the same.
See related: Comparatively Speaking; Solubility vs. Partition Coefficient










