Knowing the difference between a silicone fluid and bimodal silicone fluid is important for formulators using them. A simple specification such as viscosity will not suffice in duplicating a product that is a bimodal blend.
It is important to note that all polymers are oligomeric compositions. This means there are a series of different molecular weight polymers in the product. This is not a situation unique to silicone polymers. We see a Gaussian distribution of polymers in ethoxylates and many other common polymers.
See related: Comparatively Speaking; Chemical Compound vs. Chemical Complex Composition
The fact that polymerization results in several species that are present in a polymer because of the polymerization process should not be confused with intentionally blended silicone polymers that have two different oligomer distributions, one from each polymer. Since many silicone fluids are sold by viscosity, it is often difficult to determine if a particular product is the result of blending or a single silicone fluid without the proper analytical methodology.
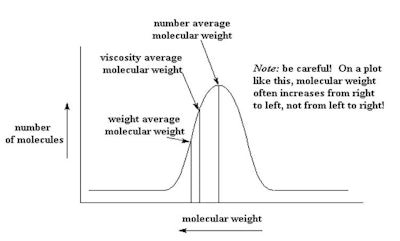
Gel permeation chromatography (GPC)1 is a typical recommended system to complete this analysis. It shows the distribution of polymeric species in a polymer (see Figure 1).
Figure 1. Typical distribution of polymer oligomers
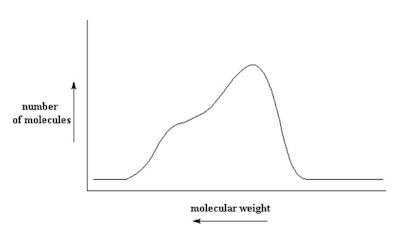
 The distribution shown in Figure 1 is a result of making the polymer and is not a bimodal distribution. It is one distribution. A bimodal distribution is the result of a blend. Figure 2 shows a bimodal distribution of two different molecular weight silicone fluids. This type of bimodal distribution results from blending a low viscosity fluid with a high viscosity fluid. Consider a blend of a dimethicone made up of a blend of 1,000 cSt and 50 cSt blended to a ratio of 350 cSt (see Figure 2). A digitized program is available in which you enter the two dimethicone fluids to be blended and the desired viscosity. Using this calculator,2 the ratio to blend is 35% 50 cSt and 65% 1.000 cSt.
The distribution shown in Figure 1 is a result of making the polymer and is not a bimodal distribution. It is one distribution. A bimodal distribution is the result of a blend. Figure 2 shows a bimodal distribution of two different molecular weight silicone fluids. This type of bimodal distribution results from blending a low viscosity fluid with a high viscosity fluid. Consider a blend of a dimethicone made up of a blend of 1,000 cSt and 50 cSt blended to a ratio of 350 cSt (see Figure 2). A digitized program is available in which you enter the two dimethicone fluids to be blended and the desired viscosity. Using this calculator,2 the ratio to blend is 35% 50 cSt and 65% 1.000 cSt.Figure 2. Bimodal blend of 50 cSt and 1,000 cSt to make 350 cSt
 Finally, bimodal silicones are commonly made by blending very high viscosity fluid and very low viscosity fluids to a target viscosity. The low viscosity fluid is added to alter the rheology of the high viscosity fluid to allow for better spreading and lower the sticky feel of the high viscosity fluid. The high viscosity, once spread onto the skin in a thin film, has a very pleasing skin feel.
Finally, bimodal silicones are commonly made by blending very high viscosity fluid and very low viscosity fluids to a target viscosity. The low viscosity fluid is added to alter the rheology of the high viscosity fluid to allow for better spreading and lower the sticky feel of the high viscosity fluid. The high viscosity, once spread onto the skin in a thin film, has a very pleasing skin feel.See also: Comparatively Speaking; Viscosity in CentiPoise (cP) vs. CentiStokes
The 100,000 cSt fluid would be as thick as hot tar. It would not spread well on the skin and would only be viable in a personal care product in a blend. The 50 cSt by itself product would be very thin and have little or no cushion. In blending the two, the 50 cSt fluid lowers the viscosity of the blend, provides for ease of spread and acts like a "delivery system" for the high viscosity fluid. The desirable properties of the high viscosity fluid are delivered in a cosmetically elegant way. Also, keep in mind that the 50 cSt, the 100,000 cSt and the blend all share the INCI name of dimethicone.
Figure 3. Bimodal blend of 50 cSt and 1,000 cSt to make 350 cSt
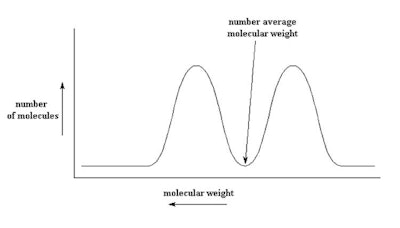 As described, the type of bimodal distribution shown in Figure 3 results from blending a low viscosity fluid with a high viscosity fluid (100,000 cSt with 50 cSt). Such a fluid would have very different functional properties than a single modal product made to the same viscosity. Interestingly, in the bimodal blend shown in Figure 3, there are no molecules having the number average molecular weight, while in the mono-modal product, the concentration of the of polymer at the number average molecular weight is very high.
As described, the type of bimodal distribution shown in Figure 3 results from blending a low viscosity fluid with a high viscosity fluid (100,000 cSt with 50 cSt). Such a fluid would have very different functional properties than a single modal product made to the same viscosity. Interestingly, in the bimodal blend shown in Figure 3, there are no molecules having the number average molecular weight, while in the mono-modal product, the concentration of the of polymer at the number average molecular weight is very high.