The sunscreen market has long been dominated by sun care products using organic UV filters. However, incorporating these chemical actives into formulae intended for day-long wear is a concern. One problem is they can cause skin irritation in some consumers. What’s worse, some people become sensitized to certain UV filters, and there is no guideline for avoiding the irritation problem beforehand.
Another challenge is the tendency of UV filters to aggregate, which can reduce their SPF performance despite increasing their use levels. To overcome this problem, formulators may mix four to six different UV filters in the formula for a total concentration of no less than 18% to produce an SPF 50 sunscreen. Such a high level of sunscreen and variety of UV filters in one formula inevitably increases the risk of skin irritation.
Considering these challenges, a specialized water-in-oil-in-water (w/o/w) system was developed to function as an isolating shield, enwrapping UV filter particles to prevent them from aggregating while at the same time helping their dispersion. With the aggregation problem solved, SPF performance is greatly enhanced, as is shown here. Further, efficiency is improved since lower levels of UV filters are required to provide higher SPFs.
W/O/W System
To prepare the system, a proprietary, high pressure and high shear process is used to control the particle size of the pre-solubilized mixture of both liquid and powder chemical sunscreen at micron meter levels. The sunscreen mixture is then encapsulated in a w/o/w double sphere system, which stabilizes the chemical sunscreen content and converts it into an aqueous dispersion (see Figure 1). This unique double sphere structure has a lipophilic active ingredient, such as a chemical UV filter, enwrapped in the outer layer. In addition, the hydrophilic active ingredient is enwrapped in the inner core.
SPF In vitro
To assess the capabilities of the w/o/w technology, two sunscreen combinations were formulated with the system for SPF testing. The first was a combination of ethylhexyl methoxycinnamate (20%), octocrylene (10%) and butyl methoxydibenzoylmethane (20%)a. The second incorporated ethylhexyl methoxycinnamate (25%), benzophenone-3 (10%) and butyl methoxydibenzoylmethane (15%)b. Both combinations were added at different percentages into a gel (see Formula 1) and cream (see Formula 2).
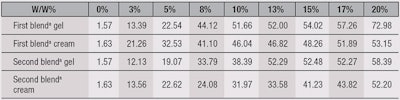
SPF performance was measured using a UV transmittance analyzerc, the results of which are shown in Table 1. As can be seen, just 10% of either sunscreen blend was required in the final formulae to achieve an SPF of 50 or more—even up to 70, in the case of the first blend in a gel formula. Further, when UV filter combinations were incorporated in the cream with 3% titanium dioxide, the SPF performance was boosted dramatically; the cream formula with TiO2 is shown in Formula 3, and the respective SPF performance is shown in Table 2.
The cream with 3% TiO2 alone yielded an SPF of only 4.88, compared with the blank cream having no sunscreen actives, which provided an SPF of 1.63. However, when 3% of the firsta or secondb sunscreen blend was added to the cream, the SPF performance increased from 8 to 11 times, easily achieving an SPF of 50 and 30, respectively. This was with a total concentration of organic and inorganic sunscreen actives at just 4.5%. Such synergistic effects have never before been observed.
UVA Protection
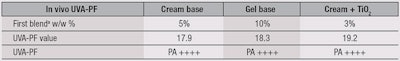
The UVA protection capability achieved by each formula containing the w/o/w system, as determined following the Boots Star Rating system, also was outstanding, as shown in Table 3. Almost all formulas achieved the highest UVA protection possible, except the first blenda at its lowest percentage. Besides the cream and gel formulas presented here, the w/o/w system has been incorporated into other formulae to test for SPF performance. All showed remarkable and reproducible SPF results; the results of another cream formula including 3% TiO2 are presented later in this article.
UVA-PF In vivo
UVA-PF in vivo testing also was performed, in accordance with the Declaration of Helsinki and national regulations regarding human studies as described by International Standard ISO 24442. Similar in vivo results were obtained to those found in vitro (seeTable 4).
Photostability
As is generally known, organic UV filters provide sun protection with more a comfortable skin feel than inorganic sunscreens. However, their UV-absorbing mechanism also flaws their protective capabilities. As Gago-Ferrero et al. explain,1 organic UV filters absorb radiation with excitation to a higher energy state. This excess energy is dissipated by the emission of higher wavelengths or energy-shedding by photochemical processes; for example, isomerization and heat release. Thus, when interacting with UV radiation, organic UV filters undergo a degradation process and lose their UV protection ability. This is why product labels remind consumers to reapply sunscreen every few hours—to maintain protection.
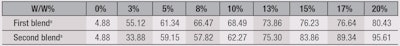
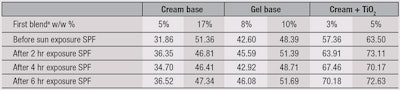
In relation, an after-sun-exposure SPF study was conducted to determine whether the UV absorbance rate of the w/o/w system decreases after being exposed to UV light. The firsta sunscreen blend was incorporated in a cream base at 5% and 7%; in a gel base at 8% and 10%; and another cream at 3% and 5% that also contained 3% TiO2. The slides to which each sample was applied were exposed to natural sunlight in the southern part of Taiwan, at noon, for up to 6 hr. After sun exposure, the SPF protection of each was tested.
Results, shown in Table 5, indicated the w/o/w technology prolonged the protective effects of the chemical UV filters. The SPF of each formula including the w/o/w technology decreased very little, even after 6 hr of sun exposure. And comparing the data in Table 3 with Table 5, the SPFs of the TiO2 cream were raised to 20~40 higher than those cream formulas without 3% TiO2. Although the exact mechanism of this protection-prolonging effect is still unknown, this technology presents interesting potential for application with chemical UV filters.
Percutaneous Absorption
As noted, one drawback to organic UV filters is they can cause skin irritation, potentially deterring the user from using products containing them. This irritation occurs because organic UV filters in conventional preparations can penetrate the skin and reach the capillaries.2-4 In response, the body’s natural defense system is initiated and reacts to the alien UV filter with rashes and swelling.
Formulators have spent long hours trying combinations of UV filters to reduce the potential for allergic reactions. In relation, the results of a percutaneous absorption study with this w/o/w technology, described here, show it could solve such problems. The test was carried out following OECD guideline 428 with some modifications.5 Franz cells (see Figure 2) and non-viable skin were used for mimicking skin absorption physiology.
Three cream samples were formulated based on cetyl alcohol, hydrogenated poly-1-decene, polyglyceryl-3 methylglucose distearate, Euglena gracilis polysaccharide, phenoxyethanol, 3-iodo-2-propynylbutylcarbamate and propylene glycol. One additionally contained octyl methoxycinnamate (OMC) alone; another contained OMC with the w/o/w system; and the third blank cream included no UV filters. All samples were applied on non-viable skin and left for 24 hr in a 36.6°C environment.
After incubation, the buffer solution from the Franz cell receptor chamber was drawn off to test its UV-absorbing capabilities. Figure 3 shows the buffer solution drawn from the w/o/w-treated OMC chamber had much less UV-absorbing capability than OMC alone. Interestingly, although the buffer solution in the OMC + w/o/w chamber showed trivial UV-absorbing capabilities, its curve was almost identical to the solution drawn from blank-cream chamber (see Figure 4).
This shows the minimal UV-absorbing capability was likely from other ingredient(s) in the cream base. From the results of this percutaneous absorption study, the w/o/w technology was found to provide a strong propensity for reducing UV filter absorption into the skin, hence reducing the irritation potential of the chemical sunscreen.
In vivo Skin Irritation/Sensitization
A third partyd tested the firsta w/o/w system for skin irritation and sensitization using a repeated insult patch test (RIPT). Fifty-two subjects, ages 20-67 and including nine men and 43 women, were enrolled. Results (not shown) indicated the sample was a non-primary irritant and non-primary sensitizer to the skin.
Conclusion
Despite the popularity of UV filters used in sun protection products, they still present challenges the industry has yet to address. The practicability of incorporating this new w/o/w form of UV filters has been widely proven, and its successful implementation into formulations welcomed by formulators. The most difficult challenges can now be resolved by remarkably enhancing the performance of well–accepted, existing organic UV absorbers. Such “old” materials are thereby renovated for easier and safer formulation, meeting the challenges faced by formulators and resulting in big benefits for the users of sunscreen products.
References
- P Gago-Ferrero, MS Diaz-Cruz and D Barcelo, An overview of UV-absorbing compounds (Organic UV Filters) in Aquatic Biota, Anal Bioanal Chem 404 2597-2610 (2012)
- Z León González, Percutaneous absorption of UV filters contained in sunscreen cosmetic products: Development of analytical methods, Springer Theses (2014)
- H Gonzalez, A Farbrot, O Larkö and A-M Wennberg, Percutaneous absorption of the sunscreen benzophenone-3 after repeated whole-body applications, with and without ultraviolet irradiation, Brit J Derm 154(2) pp 337–340 (2006)
- GJ Nohynek and H Schaefer, Benefit and risk of organic ultraviolet filters, Regulatory Toxicology and Pharmacology 33(3) pp 285-99 (2011)
- OECD, Skin absorption: In vitro method, Guideline 428, Guidelines for the Testing of Chemicals, Paris (2004)