The use of titanium dioxide (TiO2) in cosmetics is extensive; in addition to makeup such as pressed powder cakes, liquid foundations, lipsticks and eye makeup, it is used in sunscreen creams. However, its rutile form for cosmetics may produce small amounts of hydroxyl radicals upon exposure to sunlight. Therefore, in the present article, the authors use salicylic acid (SA) as a hydroxyl radical capture agent to examine the development of free radicals in solutions or creams containing TiO2 to further examine its safety upon UV exposure.
Characterizing TiO2
TiO2 is a nontoxic, fine white powder consisting of uniform particles that has good dispersion and narrow particle size distribution. Its coloration and covering power are higher than zinc oxide or lead carbonate, and for particle sizes in the range of 0.2–0.5 μm, TiO2 has the largest reflective index (n = 2.7) among all white pigments. It also shows stable chemical properties such as being: insoluble in water and weak acids, slightly soluble in alkaline solutions, and soluble in hot sulfuric and hydrochloric acid. Crystal forms of TiO2 include anatase, rutile and brookite.
The anatase and rutile forms are the most often used for pigment and photocatalytic reactions.1 These forms are tetragonal structures (see Figure 1) and their molecule bonding type and curvatures are expressed as different densities and band gap energies. When the anatase form is heated to approximately 600°C, it transforms into the rutile form. Typical TiO2 on the market is a mixture of the anatase and rutile forms doped with other metal ions.
Photocatalytic Oxidation via TiO2 Catalyst
In 1972, the photocatalytic splitting of water on TiO2 electrodes marked the beginning of a new era in heterogeneous photocatalysis.2 Since then, research efforts3 have been under way to understand the fundamental processes and to enhance the photocatalytic efficiency of TiO2. Such studies often relate to energy renewal and energy storage.
In a heterogeneous photocatalysis system, photo-induced molecular transformations or reactions take place at the surface of a catalyst. Depending where the initial excitation occurs, photocatalysis can generally be divided into two classes of processes. When initial photo-excitation occurs in an adsorbated molecule, which then interacts with the ground state catalyst substrate, the process is referred to as catalyzed photoreaction. If initial photo-excitation instead takes place in the catalyst substrate and the photo-excited catalyst then transfers an electron or energy into a ground state molecule, the process is called sensitized photoreaction.4
The crucial reaction mechanism of photocatalysis is the electron’s movement and the subsequent hole it generates between the conduction and valance bands after UV exposure. When the electron and hole recombine, photocatalysis activity diminishes. By decreasing the particle size of photocatalyst crystals, chemists effectively increase the band gap energy, which leads to a blue shift or the so-called quantum size effect. This state makes it difficult for activated electrons in the conduction band to return to the valance band, meaning fewer electron-hole pairs recombine. In relation to this, the anatase type of TiO2 provides excellent photocatalysis due to a higher band gap energy (see Figure 2).5
Anatase TiO2 has a band gap energy of 3.2 eV, and rutile TiO2 has a band gap energy of 3.0 eV. When light below a certain wavelength is incident on these crystals, electron-hole pairs are generated according to the equation: TiO2 + hν → e− + h+, where h refers to Planck’s constant (in J·s) and ν refers to frequency (in Hz), thus hv refers to the photo-irradiation; and h+ refers to the electron hole. The threshold wavelength is λ ≤ 380 nm for the anatase form, and λ ≤ 413 nm for the rutile form.
From this reaction, a variety of reactions may follow but the one of primary interest is the reaction with oxygen in air that generates free radicals, represented by the following equation.
Eq. 1 O2+ e− → [O2] •−
First, the generation of superoxide radicals occurs via the following three reactions.
[O2] •− + H2O → HO2 + OH−
HO2 + e− → [HO2]− and
[OH2]− + H2O → H2O2 + OH−
Finally, hydroxyl radicals are generated by the reaction: OH− + h+ →OH•. These radicals attack organic substrates initiating the process of photocatalytic oxidation.6 This photocatalytic effect is not obvious. However, several methods can be used to detect hydroxyl radicals; for instance, chromatography determines trapping products after their reaction with scavengers.7
In advanced oxidation, hydroxyl radicals emerge in relatively large amounts but the electrochemical Fenton reaction can be used to simultaneously reduce dioxygen and ferric ions to permit the controlled production of OH• radicals for the stepwise hydroxylation of benzoic acid to mono- and polyhydroxylated products. Therefore, the system typically is optimized for a specific pH range; e.g., pH 3-4 for Fenton’s oxidation.8
The formation of salicylate derivatives in a trapping reaction is also pH-dependent, thus HPLC can be used to examine the radicals trapped and quantitatively distribute all hydroxylated products. Overall, the reaction scheme is established and rate constants of individual steps are measured.9 To confirm whether cosmetic TiO2 yields free radicals in solution or creams, the authors thus chose SA as a hydroxyl radical capture agent (capturer or dismutant), and a conventional UV detector was used to monitor hydroxylated derivatives and SA after HPLC separation.
Materials and Methods
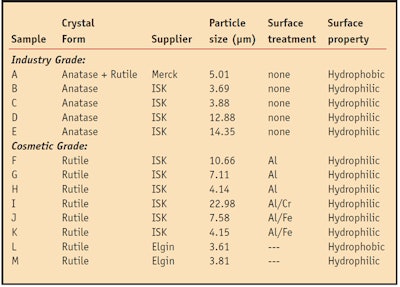
Chemicals and reagents: Thirteen commercially available cosmetic grade TiO2 samples including both the anatase and rutile type were obtained from different companies and used to test for the generation of free radicals after UV irradiation. Table 1 shows the crystal forms and particle sizes of individual TiO2 samples. By liquid-solid heterogeneous photocatalysis reaction principal, the authors used SA as a hydroxyl radical capture agent and produced catechol, 2,3-dihydroxy benzoic acid (2,3-DHBA) and 2,5-dihydroxy benzoic acid (2,5-DHBA), as shown in Figure 3.
Generation and trapping of hydroxyl radical: 1000-ppm SA was added to a 12% methyl alcohol aqueous solution, then 1%, 3%, 5% and 7% TiO2 solutions were mixed and irradiated by a 250-W UV lamp at 35°C for 6 hr. The end products were measured hourly by HPLC with a UV detector. The same method was used to test the sample w/o and o/w creams shown in Formula 1a-b.
Results and Discussion
Under the experimental conditions, a pH level of 6 was maintained, similar to human skin acidity; in addition, the reaction temperature was initiated at 35°C, also approximating that of human skin. Figure 4 shows that after the reaction a greater amount of SA was consumed from the pH 6 solution of TiO2 (sample A, Table 1) under UV irradiation.
Figure 5 shows that the TiO2 without a surface coating in solution consumed approximately 3.0% of the SA, while the same concentration of solution including a TiO2 coated with aluminum oxide or chromium oxide consumed 1.5% of SA. The authors thus concluded that cosmetic grade TiO2 reliably induces a photochemical catalysis reaction. Without surface treatment, the TiO2 was similar in reactivity to the same concentration of the photocatalyst described above. The reaction percentage of 1% TiO2 (sample A, Table 1)) was 3.08%, and at 7% it was 2.66%. Figure 6 also shows that the conversion rate quickly grew as the amount of TiO2 increased—from 4 to nearly 12 times (11.85%).
A hydrophobic coating on the surface resulted in better oil partition since TiO2 without surface treatment does not participate in the oil phase of an emulsion. However, the conversion rate of hydrophobic TiO2 rises faster than a hydrophilic version. This is because the water phase can provide electrons to reduce the free radicals generated.
As a final goal, the authors sought to identify which forms of TiO2 sunscreen generated the least amount of radicals. Figure 7 shows how, by using an o/w form of emulsion, the production of free radicals generated can be reduced to almost zero. As noted, in o/w emulsions, the hydrophobic TiO2 (sample L, Table 1) dispersed in the oil phase and the water phase protects it like a coating layer.
Conclusions
Results from testing cosmetic-grade TiO2 for the production of free radicals after UV exposure indicated a reaction toward SA, albeit unapparent, at a conversion rate of less than 3%. Results also showed that for two TiO2 samples formulated in an emulsion, the SA reaction percentage declined sharply. These dioxides formed a hydrophobic coating on the surface, thus improving the oil partition of TiO2. The most important result is that, by using an o/w emulsion, one can reduce the free radicals generated to almost zero. While TiO2 is used widely in sun care products, the recent focus has primarily been on water-repellency. Here, for safety considerations and to reduce the generation of free radicals, the authors suggest that o/w emulsions are better than dispersions of oil or water alone.
References
- Diebold, The surface science of titanium dioxide, Surf Sci Rep 48(5-8) 53-229 (2003)
- A Fujishima and K Honda, Electrochemical photolysis of water at a semiconductor electrode, Nature 238(5358) 37-38 (1972)
- AJ Bard, Design of semiconductor photoelectrochemical systems for solar energy conversion, J Phys Chem 86(2) 172-177 (1982)
- AL Linsebigler, G Lu and JT Yates, Photocatalysis on TiO2 surfaces: Principles, mechanisms and selected results, Chem Rev 95(3) 735-738 (1995)
- RPS Suri, J Liu, DW Hand, JC Crittenden, DL Perram and ME Mullins, Heterogeneous photocatalytic oxidation of hazardous organic contaminants in water, Water Environ Res 65(5) 665-673 (1993)
- MA Oturan and J Pison, Hydroxylation by electrochemically generated OH• radicals. Mono- and polyhydroxylation of benzoic acid: Products and isomers’ distribution, J Phys Chem 99(38) 13948-13954 (1995)
- A Fujishima, TN Rao and DA Tryk, Titanium dioxide photocatalysis, J Photochem Photobio C: Photochem Rev 1(1) 1-21 (2000)
- FJ Jen, MF Leu and TC Yang, Determination of hydroxyl radicals in an advanced oxidation process with salicylic acid trapping and liquid chromatography, J Chromatogr A, 796(2) 283-288 (1998)
- L Diez, MH Livertoux, AA Stark, M Wellman-Rousseau and P Leroy, High-performance liquid chromatographic assay of hydroxyl free radical using salicylic acid hydroxylation during in vitro experiments involving thiols, J Chromatogr B 763(1-2) 185-193 (2001)