During normal, infrequent, everyday use, tissue products are inherently nonirritating. However, with allergies, a cold or the flu, frequent and repetitive use can lead to inflammation of the nasal area,1 including redness, swelling, pain and heat.2 Several finished products had already been made commercially available to help prevent or alleviate wiping-induced discomfort or to soothe consumers via aromatherapy; for example, softer, lotion-infused or mentholated tissues. While these technologies help to minimize and prevent further nasal irritation and comfort the consumer, none had targeted the heat and redness associated with an already inflamed nose.
This presented a market need for which the authors sought to develop a new, more soothing tissue that actively cools a hot and sore nose upon contact. However, accomplishing such a task posed several formulating challenges ranging from ingredient selection and practical application, to efficacy and safety testing, among others. Described herein are the steps taken to find a practical solution to this product challenge.
Performance Profile
Several technical criteria were first determined for the development of the product. The formulation needed to provide a noticeable cooling sensation upon use, as well as provide a soothing benefit greater than the market standard. It also had to be compatible with the existing manufacturing process for lotion facial tissue, exhibit a safety profile equal to the market-leading lotion facial tissues, and provide appropriate consumer aesthetics. Additional criteria included: maintaining tissue parameters, i.e., strength, absorbency, etc.; formulation stability on the tissue; and cost and packaging compatibility.
Materials and Methods
Of the known technologies that can provide a cooling sensation in cosmetic products, phase-change materials were the primary candidates evaluated. A phase-change material (PCM) is solid at room temperature and provides a cooling sensation by taking advantage of a high latent heat of fusion (ΔHf) as it melts.3 The PCM absorbs thermal energy, so when it is brought into contact with the elevated temperature of a hot and sore nose, it absorbs this energy as it melts. The removal of this energy provides the cooling sensation and consequently, a soothing response to the consumer.
Of particular interest were PCMs with a melting point in the range of 75–92°F (23.8–33.3°C), since they would melt at skin temperature and provide the user a cooling sensation. PCMs were chosen as described later in this article, incorporated into test formulations (see Table 1), coated onto tissues, and evaluated by trained panelists following a proprietary soothing protocol for efficacy. The various PCMs and blended formulation samples were analyzed for cooling potential using a differential scanning calorimeter (DSC)a. DSC thermograms were recorded on 10–15 mg quantities of each sample in a sealed aluminum pan over the temperature range -50°C to 75°C, and under a dynamic nitrogen atmosphere. This protocol was repeated for several cycles and the data was recorded from the first and last cycle. The DSC instrument softwareb was used to analyze the data. The amount of crystallinity in the diluents was evaluated via light microscopy.
Trained panelists then evaluated the prototype products for cooling effects, after-feel and residue on hands. Additional clinical tests utilizing repetitive nose-wiping were also completed to assess comfort, i.e., transepidermal water loss (TEWL), redness and perception, in use during multiple wiping events and compared with market-leading lotion facial tissues. As noted, the ability of the tissue to soothe an already irritated nose was gathered from a proprietary soothing protocol.
PCM Selection
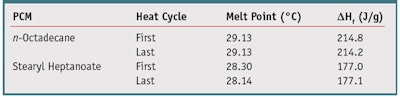
Several PCMs exist, including fatty esters, straight chain hydrocarbons, paraffin waxes and others. The latent heat of fusion for a subset of cosmetically acceptable ingredients was measured and prototype products coated with the subset of PCM ingredients were evaluated to determine their perceived cooling effects. By combining the consumer cooling perception response data along with the PCM application amount, a latent heat of fusion value greater than or equal to 150 J/g was used as a threshold for selection of the PCM. Aside from their thermodynamic feature, these PCMs were also evaluated for commercial availability and the ability to be coated onto tissue in a liquid state and solidify post-treatment without a change in the tissue manufacturing process. Based on all criteria, two PCMs stood out: n-octadecane and stearyl heptanoate. The remainder of the development focused on these two ingredients, to optimize performance and delivery.
Consistency Considerations
To ensure a consistent product, both the purity of the PCM and the reproducibility of its melting behavior are important. Since the PCM drives the cooling sensation, impurities can cause variations in behavior as the product melts and freezes, and variations in temperature during shipping and storage are expected. Therefore, the product must maintain consistent cooling effects after exposure to these conditions. The evaluation of both the purity of neat n-octadecane and stearyl heptanoate and the reproducibility of their melting behaviors was carried out using DSC over several cycles. A consistent melt point indicated a pure product and a consistent latent heat of fusion indicated reproducible cooling behavior over several melt/freeze cycles. Table 2 depicts the melt point and latent heat of fusion of n-octadecane and stearyl heptanoate for the first and last cycles. Both PCMs displayed stability over several melt cycles with no changes in melting point or separation of melting and freezing peaks in the thermogram. This indicates the PCMs would have a consistent melt and maintain cooling ability for the shelf life of the product.
To evaluate their behavior on the substrate, the two PCMs were coated onto the tissue. Light microscopy was then employed to evaluate the coating uniformity. Figure 1 shows the appearance of tissues coated with neat n-octadecane and neat stearyl heptanoate. n-Octadecane formed an uneven coating on the tissue that consisted of either large crystals or agglomerates of crystals. Stearyl heptanoate formed a more even coating across the tissue without large or agglomerated crystals.
Use Conditions
Both the stearyl heptanoate and n-octadecane coated tissues were then tested in a repetitive nose wiping (RNW) clinical study to assess comfort during use compared to market leading lotion facial tissue. The RNW clinical protocol exaggerates product use to create experiences one might encounter during a bout with allergies or a cold/flu event. Both stearyl heptanoate and n-octadecane caused discomfort when used alone under these exaggerated use conditions.
PCM Choice
As both PCMs tested were stable over several melt cycles and displayed the same results in the RNW, a choice was made to develop the stearyl heptanoate for this project. This was primarily due to its uniformity of coating on the tissue to consistently deliver the cooling benefit. Both vendor data and the material’s use in skin care products suggested that stearyl heptanoate had an acceptable skin safety profile. However, the question of why the material caused discomfort during exaggerated use remained. It is known that repetitive wiping can cause irritation, inflammation and discomfort,1 so it was hypothesized that after discomfort was initiated, the molten PCM coming into contact with the skin exacerbated this condition.
Dilution Effect
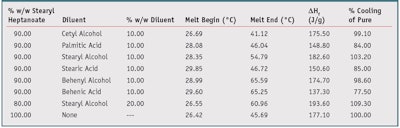
To reduce the amount of PCM in the formulation, and therefore reduce cost and potential discomfort, a decision was made to dilute the PCM with low-cost crystalline diluents that melt at a higher temperature than the PCM. This approach entrapped the PCM within the solid diluent, reducing initial skin contact. However, the final PCM concentration also needed to remain high enough to maintain cooling performance. Therefore, combinations of PCM with different fatty alcohols and fatty acids were made and their latent heat of fusion measured. Table 3 shows selected data points from this set of experiments.
The “% Cooling of Pure” column expresses the enthalpy of the blends as a percentage of the enthalpy of pure stearyl heptanoate. If the concentration of phase change material was directly proportional to the cooling ability, i.e., enthalpy of the compositions, an enthalpy of 159.39 J/g or 90% of 177.10 J/g would be expected from a 10% reduction in the full enthalpy of 100% stearyl heptanoate. As is evidenced by Table 3, several formulations retained 90% or greater enthalpy of 100% stearyl heptanoate while reducing the overall loading of the phase change material by 10–20%.
Also evidenced in Table 3, the fatty alcohols performed better than the corresponding fatty acids of the same carbon chain length. For instance, the C-18 stearyl/stearic chain length blends had a higher percentage cooling of pure stearyl heptanoate than all other materials tested in their class. It is believed that the self-assembling crystalline structure of the diluent materials provides a scaffold within and around which the PCM aggregates. This aggregation allows the crystal size of the PCM to be large enough to provide at least 75% of the enthalpy of the pure phase change component, thus providing an equivalent or near-equivalent cooling sensation to the pure phase-change material.
Crystalline Structure
Stearyl alcohol and cetyl alcohol both displayed favorable ΔHf values and retained > 98% cooling of the pure PCM; however, while preparing formulations containing these diluents, it was observed that some of the formulations contained large crystals and had a shiny, flaky appearance when coated onto a tissue. To understand the crystalline structure of these fatty alcohols, a light microscopy study was conducted. The fatty alcohol component was melted, a drop was placed on a glass slide, and a cover slip was placed over the drop before it solidified. The slide was then allowed to cool to room temperature and was examined under 20x magnification. The PCM/fatty alcohol blends were not evaluated, as the PCM melted from the heat of the microscope light source. Figure 2 displays the crystalline structure of stearyl alcohol and cetyl alcohol. Both materials formed large crystalline networks, and it was hypothesized that these networks were causing the shiny, flaky appearance of these formulations. However, a shiny, flaky tissue was not consumer-acceptable, so a solution was needed to reduce the crystal size while maintaining the cooling attributes of the tissue.
Modifying the Crystalline Structure
To reduce the crystalline size of the fatty alcohols in the formulation, an additive was needed that would be soluble with both ingredients and reduce the crystallite size, but not alter the melting point range and associated enthalpy significantly. The addition of other small molecules (fatty alcohols/acids) would simply introduce an additional source of crystallinity, so polymeric additives seemed a more feasible approach. Samples were prepared as described above and evaluated with a light microscope under 20x magnification. Figure 3 displays light micrographs of stearyl alcohol alone and with the addition of 5% polyethylene.
As evidenced, the fatty alcohol crystalline network was significantly smaller and more uniform with the addition of polyethylene. Subsequent formulations of fatty alcohol, polymer and PCMs no longer had the large, shiny crystals but rather an appearance more aligned to that of a normal lotion tissue (data not shown). These tissues also maintained an adequate cooling effect. From a technical perspective, this formulation met the criteria to proceed to consumer testing.
Consumer Acceptance
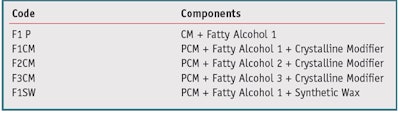
At this point, formulations from the development phase (see Table 1) required testing for comfort, appropriate cooling effects and skin feel. These compositions had the same amount of PCM but included variations of fatty alcohol carbon chain length, increasing from F1–F3; fatty alcohol concentration; and the inclusion or not of a crystalline modifier. F1SW included a synthetic wax material, commonly used in lotion tissue products, to evaluate the impact on the aforementioned parameters and the cooling perception. Tissues coated with these formulations were used in an exaggerated use clinical study to assess comfort. Test outputs included TEWL, redness as measured using the l*a*b* system, and a comfort assessment. The cooling tissues were compared to commercial lotion tissue controls. Additional samples of the coated tissues were provided to trained panelists for evaluation of cooling perception and after-feel versus a commercial control lotion tissue. Table 4 contains a summary of the results from these studies, including a plus (+) sign to indicate a favorable result and a minus (-) sign to indicate an unfavorable result.
As shown in Table 4, Samples F1 and F1CM displayed favorable cooling and after-feel characteristics whereas the trained panelists did not experience sufficient cooling with samples F2CM, F3CM and F1SW. The three samples without adequate cooling were undesirable due to that data alone; however, this trend continued with the TEWL, redness and comfort assessment data from the exaggerated use study. Formulas F2CM, F3CM and F1SW did not meet the acceptance criteria and were discontinued. Sample F1, with just the fatty alcohol and the PCM, did well in TEWL and redness but was not comfortable during use. Fortunately, F1CM performed well with trained panelist evaluations and all consumer acceptance testing.
Soothing
With the comfort and cooling performance of test product F1CM established, a proprietary soothing study was initiated to determine the consumer’s holistic response of the soothing attributes, as compared to market leading lotion facial tissue controls. Figure 4 displays the results of this study. The column marked with the asterisk (*) is significantly different at a 95% confidence level using a one-way analysis of variance. The interpretation of this data confirms that F1CM was judged as more soothing than either market control 1 or market control 2.
Conclusion
Formulation chemistry is not just about putting products on the shelf, it is about transforming a simple task into an emotionally pleasant experience. The development and commercialization of this formulation into a tissue that cools and soothes a sore nose on contact demonstrates the potential to deliver innovation and consumer delight in a mature product category. The application of PCM technology to facial tissue required physical chemistry, formulation and product development expertise to deliver F1CM into a viable business proposition. Launched in September 2011, the Kleenex brand Cool Touch tissues bring a new sensation to facial tissue users and was recognized as a finalist in the Cosmetics & Toiletries R&D Awards for the most creative application of a technology.
References
Send e-mail to [email protected].
- A Farage, Assessing the skin irritation potential of facial tissues, Cutaneous and Ocular Toxicology 24 125–135 (2005)
- MT Madigan, JM Martinko and J Parker, in Brock Biology of Microorganisms, 10th edn, Pearson Education Inc, Upper Saddle River, NJ, 752 (2003)
- MF Demirbas, Thermal energy storage and phase-change materials: An overview, Energy Sources, Part B 1:85–95 ISSN: 1556–7249 (2006)