Wrinkling is the expression of life and an inherent part of skin aging. Wrinkles hold memories of emotions, expressions and lifestyle. If only the worried frown lines could be filtered out to preserve just the smiling fine lines. It is clear that wrinkles cannot merely be wiped away but scientists can design new technologies capable of reducing fine lines; first, however, the process of aging must be understood.
Skin Aging and Wrinkle Formation
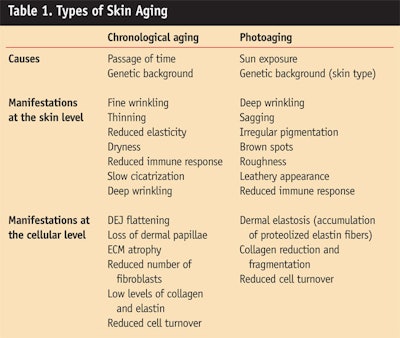
Aging is a complex process that translates into morphological and functional changes within the skin (Table 1). Skin aging occurs in two different ways that interact: the intrinsic form of chronological aging and the extrinsic form of photoaging.
Chronological aging comes with the passage of time and is influenced by individual genetic makeup. Clinical manifestations at the skin level include fine wrinkling, thinning, reduced elasticity, dryness, a reduced immune response and slow formation of scar tissue, known as cicatrization. The hallmark of aged skin is a flattening at the dermal-epidermal junction (DEJ) with loss of the dermal papillae. Also seen are a reduced number of fibroblasts as well as a general atrophy of the extracellular matrix (ECM) comprising structural proteins and glucosaminoglycans (GAGs).
Photoaging of the skin is mainly caused by chronic sun exposure. Clinical manifestations include deep wrinkling, sagging, irregular pigmentation, brown spots, coarseness and a leathery appearance. As with chronological aging, sun exposure also affects immune response in the skin. The main characteristic of photoaged skin is dermal elastosis with the accumulation of agglomerates of useless denatured elastin fibers beneath the DEJ. The collagen content is decreased and fragmented.
Wrinkles form when a force is applied to a thin, rigid material that rests on a softer, thicker basis.1 This description applies to human skin that consists of a rather rigid and thin epidermis laying on a deeper viscoelastic dermis. Wrinkles form in the skin in response to the application of mechanical constraints like muscle contraction or gravitational forces. As long as skin extensibility and elasticity are optimal and there is good cohesion between the epidermis and the dermis, wrinkles relax upon dissipation of the applied force. However, with the passage of time, cumulative alterations affecting both the structure and the mechanical properties of the skin contribute to the development of permanent wrinkles. Working on skin structure to reinforce mechanical properties thus appears to be a good strategy to ease wrinkles.
A major structure involved in skin cohesion is the DEJ (Figure 1). This is where the epidermis meets the dermis for dynamic exchanges. Composed of a complex network of interconnecting proteins, the DEJ forms the skin basal membrane. The DEJ is unique in that it holds structures forming anchoring complexes that assure mechanical stability to the skin and serve to increase frictional resistance to externally applied forces.2 Better resistance results in fewer wrinkles. Any weakness at the DEJ has tremendous effects and in some tragic pathological cases may even lead to severe skin blistering and separation of the skin layers.
As part of anchoring complexes (Figure 1), laminins, fibronectins and collagen VII are found at the DEJ. Laminin-5 is the real anchor point between the epidermis and the dermis.3 The glycoprotein bridges basal keratinocytes with collagen VII of anchoring fibrils at the DEJ. Reduced deposition of laminin-5 with age contributes to the disorganization and the flattening of the DEJ that underlies wrinkle formation.
Collagen VII is a subtype of collagen and a key structural component of anchoring fibrils at the DEJ.2 Attached to the basal membrane, these fibrils extend into the dermis to loop back, forming arcs that support dermal collagen fibers. Collagen VII plays a major role in the mechanical stability of the DEJ. Its expression is known to be reduced with aging, contributing to wrinkle formation.4
Fibronectin is a major adhesive protein whose role is to firmly anchor cells to extracellular materials.5 Through its interaction with integrins receptors at cell membrane, fibronectin also affects skin cell functions such as gene expression and cell growth. Fibronectin production is modulated by growth factors whose expression and responsiveness may be downsized with aging.
Cell matrix interactions at the DEJ are lowered with aging. Cells need a proper matrix support to survive, to stay in place, to proliferate and to differentiate and this is one of the functions of the ECM at the DEJ. Unfortunately, aging is associated with a low level of expression and an increase destruction of supporting fibers. This results in a slowing of cellular turnover and regeneration.
The structure of the DEJ is weakened and flattens. These structural changes reduce the surface area for nutritional exchange and metabolic byproducts evacuation between the dermis and the epidermis. As a consequence, epidermal cell turnover slows down even further and harmful free radicals and metabolites accumulate. Protecting and restoring cell-matrix interactions at the DEJ ensure improved, younger looking skin. Biomimetic peptides have proven to be useful cosmetic actives for that purpose.
Biomimetic Peptides in Cosmetic Precision
Traditionally, the sources of cosmetic actives have been limited to plant extracts, essential oils and vitamins; however, biomimetic molecules can now be engineered for this purpose. The beauty of the process is that highly specific ingredients that mimic natural sources can be designed with increased efficacy and specificity, requiring lower dosages. Based on the industry’s developing knowledge of the complex physiology of the skin, peptides are created and shaped in such a way that they can be substituted for natural skin factors to address various cosmetic needs. Peptides can be engineered to mimic the three-dimensional structure of beneficial skin factors, allowing them to act as such.
For example, beginning with the known sequence of a growth factor involved in wound healing, the authors produced a bank of peptides by solid phase organic synthesis. To optimize skin penetration, peptides were coupled to various lipids. Peptide conjugates were then screened in vitro for their ability to stimulate the production of structural proteins by fibroblasts at the DEJ. The selected peptide, caprooyl tetrapeptide-3a, was clinically tested in a serum formulation for fine line and wrinkle reduction.
Caprooyl Tetrapeptide-3 on Skin Proteins
In vitro studies: Confluent normal human dermal fibroblasts (NHDF) were cultured for 48 hr in the presence and absence of 10-7M (0.03%) or transforming-growth factor (TGF-b). The latter, a general stimulator of the synthesis of extracellular matrix (ECM) proteins, was used as a positive reference.6 At the end of the incubation period, laminin and fibronectin content were quantified in the supernatant using a highly sensitive and specific enzyme immunoassay (EIA) kit.
In the presence of caprooyl tetrapeptide-3, NHDF cells increased their expression of laminin by 26% and that of fibronectin by 60% (Figure 2). In the same conditions, the stimulating potential of TGF-b was 10% for laminin and 64% for fibronectin.
Ex vivo model of skin aging: An additional bench study was carried out using human skin explants exposed to corticoids as an accelerated ex vivo model of skin aging. Topical corticoids are infamous for inducing skin atrophy and flattening the DEJ, at least partly through an effect on collagen turnover, mimicking aging.7
Four human skin specimens were obtained from different patients undergoing plastic surgery and maintained in culture. Before the experiment began, the corticoid cream betamethasoneb (0.05%) was applied at the surface of some of the skin explants, leaving some explants without the cream. On the same day, caprooyl tetrapeptide-3 (0.03%) was added to the culture media of skin explants. On Day One, caprooyl tetrapeptide-3 (0.03%) treatment was repeated. On Day Three, all skin explants were frozen. The presence and localization of collagen VII and laminin-5 were assessed using indirect immunofluorescence. Visual scoring was performed by two dermatologists.
Both collagen VII and laminin-5 expressions were localized at the DEJ (Figure 3). Results revealed decreases of 16% and 45% respectively in collagen VII and laminin-5 staining in skin explants stressed with corticoids, compared to control level. Treatment with caprooyl tetrapeptide-3 in the presence of corticoids resulted in increases of 34% and 49% respectively in collagen VII and laminin-5 staining compared to what was seen with corticoid treatment alone. Interestingly, the flattening observed at the DEJ with corticoid treatment was prevented with the application of caprooyl tetrapeptide-3.
Clinical Antiaging Properties of Caprooyl Tetrapeptide-3
Antiwrinkle efficacy study: An in vivo study was conducted on 27 women ages 40 to 65, with healthy skin. Volunteers applied a placebo serum (Formula 1) on the crow’s-feet of one randomized temple area and the same formulation containing 2.5% caprooyl tetrapeptide-3 on the crow’s-feet of the other temple. Applications were repeated twice daily for 56 days. The micro relief of the eye area was assessed using special processing software from scanning by interference fringe profilometry of silicone replicas taken at days 0, 28 and 56.
Replicas appearing in as little as 28 days of application (Figure 4) show a significant average reduction in fine lines and wrinkles of 16% in the presence of caprooyl tetrapeptide-3—with a maximum of 29%. Interestingly, for individuals aged 50–65, benefits continued to progress over two months for an average reduction in fine lines and wrinkles of 27% with a maximum of 35% (Figure 5).
Ultrasonographic study: To further document the in vivo effect of caprooyl tetrapeptide-3 on dermal structures, echographic measurements were taken. High-frequency ultrasound imaging known as echography can be used to identify the dermis that is visualized as a speckled pattern.8 The echoes of the dermis are considered to originate from the boundaries between collagen and elastin fibers, the surrounding water-rich ground substance and cells.9
Changes in echogenicity may reflect an altered connective tissue composition of the dermis. In particular, appearance of a superficial low-echogenic band in the dermis, immediately below the epidermal entrance echo, has been related to age.10, 11 Dermal echogenicity has therefore been proposed as a marker for skin aging.
All 27 women volunteers, ages 40–65, applied a placebo serum on the crow’s-feet of one randomized temple area and the same formulation containing 2.5% caprooyl tetrapeptide-3 on the crow’s-feet of the other temple. Applications were repeated twice daily for 168 days. Skin echography scanning was performed on temple areas at Day 0 and Day 168. A 20-MHz ultrasound scannerc was used to obtain cross-sectional images of the skin. This process involved an echography technique allowing for an image to be taken in two dimensions known as B mode or brightness on display.”
Results showed that caprooyl tetra-peptide-3 treatment had a positive influence on skin connective tissue. As shown in Figure 6, application of the peptide for six months restored the echogenic response of the upper dermis lost with aging. Most notably, the ultrasound parameter “entropy” significantly improved in the treated area versus placebo for 78% of the volunteers. Entropy represents the disorder of an image that increases with better skin hydration.
Consumer opinion: The objective of this study was to evaluate the consumer perception of caprooyl tetrapeptide-3 as an anti-wrinkle active following
56 days of treatment. A total of 30 women volunteers, ages 40–65, participated in the self-assessment study. Each consumer applied a serum of 2.5% caprooyl tetrapeptide-3 twice a day on the crow’s-feet of the temples for a period of 56 days. Subjects were asked to fill out a self-evaluation questionnaire at the end of the study in order to evaluate their overall opinion and feeling on the effectiveness of the product.
Results condensed on Figure 7 revealed that consumers agree that caprooyl tetrapeptide-3 renders the skin smoother, firmer and more even for a younger and more rested look. Moreover, caprooyl tetrapeptide-3 provided an immediate well-being effect and was well-tolerated around the eye.
Conclusions
Biomimetic peptides are effective instruments to restore and support skin physiology, as the authors have shown here. They mimic natural skin factors and have the potential to revive signaling pathways that have lost responsiveness with age. In the present report, evidence was presented supporting the use of the growth factor-derived peptide caprooyl tetrapeptide-3 for the reduction of fine lines and wrinkles.
In vitro, caprooyl tetrapeptide-3 stimulated the production of laminin and fibronectin by fibroblasts at a similar or greater level than TGF-b used as a positive control. Ex vivo treatment of skin explants with caprooyl tetrapeptide-3 in a model of corticoid-induced skin aging resulted in the protection of laminin-5 and collagen VII expression at the DEJ. While expression of these proteins was seriously impaired with corticoid treatment alone, DEJ flattening in this model of skin aging was also prevented in the presence of caprooyl tetrapeptide-3.
In clinical trial, caprooyl tetrapeptide-3 serum significantly and rapidly reduced the appearance of fine lines and wrinkles in mature skin. Interestingly, the strength of caprooyl tetrapeptide-3 seems to self-adjust to the skin needs, delivering increasing benefits with increasing age. An ultrasonographic study additionally showed that use of caprooyl tetrapeptide-3 over six months reduced the appearance of a low echogenic band that develops in the upper dermis with age. Improved echogenicity means better collagen content and integrity for improved water retention within the dermis, translating into a younger skin appearance. Volunteers also valued caprooyl tetrapeptide-3 as an antiaging agent.
Ultimately, this study demonstrates that supporting the production of structural proteins involved in skin cohesion at the DEJ is an effective strategy to provide a long-term, significant reduction in the appearance of fine lines and wrinkles in mature skin. In the end, controlling one’s level of wrinkling may not be so unrealistic after all.
References
1. J Genzer and J Groenewold, Soft matter with hard skin: From skin wrinkles to templating and material characterization, Soft Matter 2(4) 310–323 (2006)
2. RE Burgeson and AM Christiano, The dermal-epidermal junction, Curr Opin Cell Biol 9(5) 651–8 (1997)
3. M Aumailley and P Rousselle, Laminins of the dermo-epidermal junction, Matrix Biol 18(1) 19–28 (1999)
4. NM Craven, RE Watson, CJ Jones, CA Shuttleworth, CM Kielty and CE Griffiths, Clinical features of photo-damaged human skin are associated with a reduction in collagen VII, Br J Dermatol 137(3) 344–50 (1997)
5. KA Bush, BR Downing, SE Walsh and GD Pins, Conjugation of extracellular matrix proteins to basal lamina analogs enhances keratinocyte attachment, J Biomed Mater Res A 80(2) 444–52 (2007)
6. Y Jiang, DW Cheng, ED Crook and LP Singh, Transforming growth factor-beta1 regulation of laminin gamma1 and fibronectin expression and survival of mouse mesangial cells, Mol Cell Biochem 278(1–2) 165–75 (2005)
7. S Schoepe, H Schacke, E May and K Asadullah, Glucocorticoid therapy-induced skin atrophy, Exp Dermatol 15(6) 406–20 (2006)
8. SM Milner, OM Memar, G Gherardini, JC Bennett and LG Phillips, The histological interpretation of high frequency cutaneous ultrasound imaging, Dermatol Surg 23(1) 43–5 (1997)
9. S Richard, B Querleux, J Bittoun, O Jolivet, I Idy-Peretti, O de Lacharriere and JL Leveque, Characterization of the skin in vivo by high resolution magnetic resonance imaging: Water behavior and age-related effects, J Invest Dermatol 100(5) 705–9 (1993)
10. K Tsukahara, Y Takema, S Moriwaki, T Fujimura, T Kitahara and G Imokawa, Age-related alterations of echogenicity in Japanese skin, Dermatology 200(4) 303–7 (2000)
11. JM Waller and HI Maibach, Age and skin structure and function, a quantitative approach (I): Blood flow, pH, thickness, and ultrasound echogenicity, Skin Res Technol 11(4) 221–35 (2005)