In Malaysia, palm kernel oil is obtained as a co-product of the production of palm oil with the general ratio of palm oil : palm kernel oil being 10:1.1. Due to both increased demand for oils and fats and to the success of natural trends in cosmetics, attempts are being made to increase the production of these oils by improving plantation practices and extraction methods and introducing new varieties of palms; in fact, one new variety of oil palm was introduced in 2002 that increased the yield of liquid oil and palm kernel oil to 30%.1
Palm kernel and coconut oil contain lauric and myristic triglycerides, as well as triglycerides of other fatty acids, including capric, caprylic, caproic, palmitic, stearic and oleic. With the increasing demand for lauric and myristic acids from the oleochemical industry as feedstock to produce surfactants, the oleochemicals industry typically splits the lauric oils, whereby the fatty acids of C10 and below are removed (stripped) and the C12-14 are distilled off. The by-product containing C16, C 18 and C18:1 fatty acids is usually fractionated to yield C16-18 and C18:1 fractions.
Dihydroxystearic Acid (DHSA)
The two least economic fractions are those containing C18:1 and C16-18 triglycerides since their worldwide production is limited. The former offers interesting possibilities since it contains an unsaturated moiety, meaning its structure offers an available site for further chemical modifications. By converting C18:1 to epoxide via peracetic acid or performic acid routes, followed by hydrolysis, 9,10-dihydroxystearic acid (DHSA) is obtained (Figure 1).2, 3
Besides the reactive carboxylic group, the characteristic structure of DHSA contains two vicinal alcohol groups (glycol structure). It behaves as a long-chain fatty acid but at the same time is very hydrophilic when compared to stearic acid. Moreover, it is able to establish multicentered physico-chemical bonds with polar surfaces. Such a structure is able to orientate in space and establish hydrophilic and hydrophobic domains, leading to many interesting applications.
For instance, it changes the properties of oily phases and wax gels significantly by modifying their spreading and flow characteristics. Moreover, in experiments combining pigments and fillers described here, its interaction with their surfaces is evident because it improves color development, skin adhesion and pay-off. The polarity of DHSA, being at two molecular sites, provides unusual molecular arrangements. Indeed, even if DHSA is water-insoluble, it crystallizes with one water molecule, suggesting strong polar interactions with polar sites of other molecules and surfaces.
Many derivatives also can be formed through substitutions onto one, two or all three of the reactive sites of DHSA. Some alkyl esters obtained via reaction at the carboxyl site have been studied4,5 and show exceptional skin feel and powder-binding capability. Metallic soaps, i.e. calcium, magnesium, zinc and aliminium salts of DHSA, show pigment-coating capability and a different set of properties than standard metallic stearates.
DHSA itself can also function as a pigment-coating ingredient. Thus, work is currently under way to develop and define the various properties of DHSA for application in several types of makeup products such as foundations, mascara and lipsticks, and emulsions.
DHSA in Cosmetics
DHSA is a tasteless, off-white powder with a slightly acidic scent. With a molecular weight of 316, it is non-irritating to the skin.6 The material exhibits a melting point < 95°C when containing one mole of water; it is water-insoluble but dissolves in hot ethanol, isopropanol, acetone and lipids.
DHSA also is compatible with most ingredients. Its lipophilic behavior, coupled with good polarity, allows its use as an ingredient in color cosmetic formulations and as a coating agent for pigments. DHSA-coated pigments can improve the spreading and shine of finished cosmetic products, as determined by a trained panel. Such parameters may be related to the modification of surface wetting and oil uptake due to the coating tails of DHSA, which form a triple core structure.
Nearest to the pigment surface is the carboxyl group with an attached polar C8 chain, then a polar core at the level of the two hydroxyl groups externally surrounded by the short residual, non-polar 8 carbon atom chain. Moreover, the oil adsorption and absorption power of powders incorporating DHSA is reduced7-9 and the material can be used as an additive to improve the transparency, foaming and cleansing power of transparent soap formulations.10
Application in Lipsticks
Hydrophobically coated, stable ionic pigments are obtainable by precipitation or physical coating techniques. In the latter case, consistently coated pigments were obtained by dissolving DHSA in alcohols or acetone at 70°C, blending the solution with pigments (DHSA/pigments = 10% w/w), and evaporating the solvent while mixing. Consistency was verified by analyzing the IR spectrum of the prepared coated pigments with a finely dispersed blend of the coating agent and the pigment.
In addition, the application properties of finished products (lipsticks and mascara) prepared by such a blend were compared to those of the same products prepared by the coating technique. Precipitation pigments can be prepared using salts of divalent metals such as zinc, calcium, magnesium and trivalent metal aluminium. Indeed, DHSA has many polar sites that can establish coordination bonds with the ionic surface of metallic pigments; in other words, it is a poly-toothed structure.
DHSA and DHSA-coated pigments were added to lipsticks and lip glosses as thickening agents and shine aids, to mascara as spreading agents, and to blush formulations as binders; an example blush is shown in Formula 1.
Properties were identified by direct sensory evaluation (via expert panel) and a consumer panel comparison of formulae with and without DHSA or DHSA-coated pigments.
Lipstick Evaluation
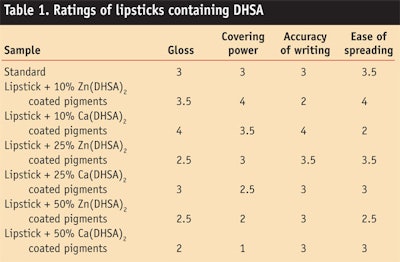
Wax gel formulas containing DHSA and standard formulas not containing DHSA were compared; these formulas were rated on a point scale from 1 (low) to 4 (optimum) by four trained panelists. Sensory properties evaluated included shine, ease of spreading, accuracy of writing, and covering power. Table 1 shows the average scores obtained.
The initial lipstick with non-coated pigments provided good sensory characteristics. Nevertheless, improvements were perceived in lipsticks containing pigments treated with Zn(DHSA)2 and Ca(DHSA)2 at 10% w/w of the pigment, as shown in Figure 2. Overall, the addition of DHSA improved pay-off, i.e., the trace amount left onto the skin during application, and ease of application while increasing mechanical resistance.
Interestingly, each metal used for DHSA precipitation and the amount of coating influenced the application properties, likely due to the varying arrangements and crystallization structure for the coating layer. For instance, the lowest amount of Zn DHSA coating provided a braked flow, which almost disappeared at a higher coating percentage. The calcium coating provided a precise trace on the skin at low coating amounts (10%), which were slightly reduced at higher coating percentages. Images taken by electron microscope show important differences in the coating arrangements (see Figure 3).11 Visually, DHSA-coated pigments also improved the shine and color-fastness of lipsticks.
Pigments coated with the zinc salt of DHSA eliminated the “motorboat” effect likely because they provided better coordination of the oils in the wax phase. This so-called motorboat effect is a phenomenon that can be observed when applying lipstick onto the forearm; the central part of the trace is normal, while at the sides two raised fluid bands are formed, like a motorboat crossing water. This is indicative of poor coordination of some of the oils by the wax gel matrix and can be corrected with the proper formulation. In fluid lip glosses, Zn-DHSA-coated pigments noticeably improved pay-off and trace homogeneity (data not shown).
Cosmetic Emulsions
In cosmetic emulsions, water is used as the emulsion phase and an evaporating ingredient to allow for adjustments such as controlled evaporation, ease of spreadability, sweat- and tears-fastness, elasticity and film-forming properties—key challenges in formulating. In cosmetic emulsions that require highly qualified spreading performance, coatings such as those afforded by DHSA can make a remarkable contribution to ease the distribution over the skin and provide homogenous results. Formula 2 shows an example of mascara formulated with DHSA-coated pigments to make eyelashes appear thicker and longer.
This mascara curled eyelashes efficiently and effectively without stiffening them, as evaluated qualitatively by a trained sensory panel. The surface of the granules is modified (see Figure 4) by DHSA such that spreading of the mascara is noticeably improved and the apparent size of lashes is also increased; moreover, the drying time was improved. Thus, the use of DHSA-coated pigments in mascara noticeably improved the curling effect while reducing the drying time of the applied layer. Pigments coated with Zn-DHSA also improved the film-forming and volumizing effects of mascara applied to the eyelashes, as observed by a sensory panel (data not shown).
Octyl Dihydroxystearate (ODHS)
Octyl dihydroxystearate (ODHS) (Figure 5) is one of the many ester derivatives of DHSA. It is produced through enzymatic esterification of palm-based DHSA with n-octanol.4,5 ODHS is a white, colorless and tasteless compound made of lustrous crystals, with a melting point of 69.9ºC. Thus, it is a soft powder with exceptional skin feel, providing a silky touch when spread over the skin surface.
ODHS in Facial Powders
In facial powders, binders are necessary to wet the surface of the powder granules to provide easy flow and lubrication among the granules. Since they possess greasy properties, binders are generally used in very low concentrations (2% to 10%). Minimizing the use of lipids helps to reduce the formation of dust that occurs during application, as well as establishes good adhesion between superficial skin lipids and the pigmented powder. Moreover, application of such a layer reduces the drying of skin since powder absorbs moisture from the skin; it also reduces the transport of sweat and sebum into the facial powder. Using a low percentage also does not allow the binder to be “squeezed out” from the granules of the compact cake, avoiding the formation of hardened plaques.
The distribution process of the binders onto powder particles is made easy by the abundant use of talc in formulations since talc provides high affinity for lipids. High chemical reactivity exists in such a system, including: very extended surfaces in the presence of pro-oxidant metallic ions; high amounts of oxygen adsorbed onto pigment and filler surfaces; milling procedures carried out in turbulent air, etc.; thus hydrocarbons and saturated lipids are the preferred oil category because of their resistance to oxidation processes. The application of ODHS in a compact eye shadow with a high content of mica and pearls is shown in Formula 3.
ODHS thus provides advantages for various cosmetic formulations, and in many tested formulations, it was found to act as a thickening agent.12 The uniformity of the applied traces of compact products were improved, together with a rich, silky and non-oily feel on the skin, as indicated by sensory panel (data not shown). In addition, ODHS increased product adhesion and lasting effects on the skin, as comparative sensory laboratory evaluation showed.
DHSA as Feedstock for Other Derivatives
As noted, since DHSA contains two vicinal alcohol groups, many derivatives can be formed (see Figure 6, Figure 7 and Figure 8), including DHSA-ethoxylates, DHSA-alkanolamides13 and DHSA-estolides.14,15
These derivatives have the potential for use in a variety of applications including as corrosion inhibitors, Gemini surfactants, cosmetic ingredients, and several yet to be explored.
Conclusions
DHSA and its derivatives are created from renewable sources and provide sensory-related and technical applications for cosmetics, especially those requiring pigments and solid fillers. Overall, better performance relating to oil phase thickening, skin adhesion, pay-off and skin feel properties were observed. The presence of polar groups allows DHSA to easily adhere to solids. Stable deposition can be improved by converting DHSA into metallic soaps through divalent and trivalent metal salts; these metallic soaps show remarkable new behaviors, the best of which are demonstrated by Zn- and calcium-based soaps. Indeed, these ingredients could find further industrial applications. In general, metallic soaps find huge industrial application as lubricants in plastics molding, in polyurethanes expansion, etc. The existence of polar hydrophilic groups in the DHSA structure could provide special water coordination properties.
DHSA is also a potential feedstock for various derivatives through substitution reactions onto one, two or all three of the reactive sites. Some alkyl esters from DHSA have been studied, which show exceptional skin feel and powder binding capability. Many more derivatives such as DHSA ethoxylates, estolides, alkanoamide are now being looked at to exploit all the possible applications of DHSA in cosmetic and personal care applications. The potential of this molecule resides in the multiple polarity sites in its structure that could provide the formulator with unexpected performance when compared to the nearest well-known compound, stearic acid.
References
1. Y Basiron and S Ahmad, Palm oil, coconut oil and palm kernel oil: Issues and future prospects, proceedings from the 2002 World Oleochemical Conference, Barcelona, Spain (Apr 14-17, 2002)
2. Malaysian patent application, PI 9804456RA, Preparation of dihydroxystearic acid from oleic acid, A Salmiah and YB Kang (1998)
3. A Roila and A Salmiah, A dihydroxy acid derived from palm-based oleic acid, proceedings of Oleochemicals Conference, PIPOC 200 90-96 (Aug 20-22, 2001)
4. A Roila and A Salmiah, Synthesis and characterization of ester from dihydroxystearic acid,
J Oil Palm Research 12(1) 81–85 (Jun 2000)
5. A Roila, B Mahiran, A Salmiah and AB Salleh, Enzymatic esterification of dihydroxystearic acid, Inform J9248 in JAOCS (11) 77 609–612 (2000)
6. Z Aldrin, R Ismail and S Ahmad, Safety evaluation for dermal and ocular irritaion of palm dihydroxystearic acid as a cosmetic ingredient, J of Palm Oil Research, 17 160–167 (Dec 2005)
7. LM Rigano, Use and advantages of palm oil derivatives in decorative cosmetics, proceedings of Oleochemicals Conference, PIPOC 2003, Putrajaya, Malaysia 7-13 (Aug 24-28, 2003)
8. R Ismail, Z Ismail, S Ahmad, R Awang and L Rigano, Di-hydroxystearic acid, a new palm oil derivative and its esters: Properties and application in decorative cosmetics, poster presented at IFSCC 2004, Orlando, FL (Oct 2004)
9. Z Ismail, S Ahmad, R Awang, R Ismail and L Rigano, Palm-based di-hydroxystearic acid in color cosmetics, paper presented at WOC OFIC (2004)
10. Malaysian patent application, PI 20082058, Transparent Soap, N Ahmad, R Ismail and HA Hasan (2008)
11. L Bettiol, Preparation and characterization of inorganic pigments superficially modified with metallic salts of DHSA and their application in decorative cosmetics, University of Trieste Thesis (2004)
12. SK Chua et, Palm-based octyl dihydroxystearate for cosmetic products, paper presented at PCHi 2006, Mumbai, India (2006)
13. A Roila, KW Cheong, B Mahiran, I Rosnah, G Razmah and A Salmiah, Alkanolamides from 9,10 dihydroxystearic acid, JOPR 18 231–238 (Jun 2006)
14. Malaysian patent application, PI20050185, A process for producing estolides, A Roila, A Salmiah, A Nor Azizan and WMZ Yunus (2005)
15. A Roila, A Nor Azizan, A Salmiah and WMZ Yunus, Characterisation of estolides from dihydroxystearic acid, JOPR 19 350–355 (Jun 2007)