Repair, protection and moisturization are key consumer needs in skin care, and components that are used to deliver against these product claims are often electrolytic in nature. Common examples include mono- and multi-valent electrolytic salts of materials such as alpha hydroxy acids, pyrrolidone carboxylic acid (PCA) and phenylbenzimidazole sulfonic acid (PBSA).1 In addition to performance against product claims, though, overall consumer acceptance is heavily swayed by product aesthetics. Sensory performance is an important parameter that provides indulgence to the user and can convey the perception of efficacy. However, balancing rheology and stabilization properties with aesthetic properties in high actives-containing formulations is a common challenge faced by the formulator.
It is well-understood that electrolytic actives can suppress the viscosity of formulations thickened with ionic rheology modifiers, and while nonionic and even slightly anionic polymers such as polysaccharides are inherently more electrolyte-tolerant, they are intrinsically less efficient and known to exhibit unpleasant textures and undesirable sensory properties, such as stickiness.2, 3 Therefore, in order to perform the required emulsification, co-emulsification, stabilization and rheology modification in oil-in-water emulsion systems but with improved electrolyte tolerance, a polymera based on an anionic pre-neutralized acrylate copolymer was developed by inverse emulsification. Referred to hereafter as the IE-acrylate copolymer, its properties and capabilities were assessed, as described.4–6
Inverse Emulsification Acrylate Copolymer
As noted, the unique structure of the liquid IE-acrylate copolymer was obtained by inverse emulsion polymerization of partially neutralized acrylic acid and ethoxylated alkyl methacrylate aqueous solution pre-emulsified in the light carrier oil hydrogenated polydecene. Polymerization occurred in the presence of cross-linker within the water droplets of the water-in-oil emulsion. Post-polymerization, the hydrophilic surfactant lauryl glucoside was added to produce an inverse emulsion. This hydrophilic surfactant aided to invert the w/o emulsion supplied to an o/w emulsion when used, allowing the water-swellable polymer to reach the continuous water phase of a skin care emulsion with mixing.
Once the water-swellable polymer reaches the bulk water phase of the intended formulation, the osmotic pressure due to counterions associated with the dissociated ionic groups of the polymer, coupled with intrapolymer repulsion due to the ionic charge, leads to rapid thickening and rheological modification. This incorporation of the hydrophobic comonomer into the cross-linked structure, in addition to the presence of low levels of high hydrophilic-lipophilic balance (HLB) emulsifier, allows the polymer to deliver efficient stabilization and to emulsify a variety of oil phases used in skin care.
Materials and Methods
All viscosity measurements presented in this article to assess the thickening and electrolyte tolerance of the test polymers, in aqueous dispersion or in emulsion, were made at 25°C, 24 hr after their production at RT and using a viscometerb. To evaluate the properties and efficacy of the IE-acrylate copolymer, several initial characterizations were first made.
Thickening: Thickening is the primary function of rheology modifiers, which change the physical properties of a system and the migration rate of suspended components. Viscosity measurements, shown in Figure 1, were made on an aqueous dispersion of the polymer, compared with other commercially available inverse emulsion polymers and thickeners selected for benchmarking purposes, at different concentrations of polymer solids. At dispersion pH and lower polymer solid concentrations, the IE-acrylate copolymer provided the highest thickening performance among the materials tested and showed exceptional viscosity efficiency.
Electrolyte tolerance in aqueous dispersion: Figure 2 shows the viscosity of 1% w/w polymer solids in the pH range of 5.5–11. The IE-acrylate copolymer provided excellent and consistent viscosity around 45,000 mPa•s above pH 6.5 but showed less thickening efficiency at pH 5.5-6.5. Since electrolyte tolerance is directly related to viscosity performance, these two specific pH ranges must be considered.
Electrolyte Tolerance at > pH 6.5
Sodium pyrrolidone carboxylic acid (NaPCA), a monovalent salt and the basic component of natural moisturizing factor (NMF), is commonly used in skin care applications. Also, zinc PCA (ZnPCA), a divalent salt, is becoming popular as an active to suppress sebum and for anti-aging products. The viscosity provided by 1% w/w polymer solids IE-acrylate copolymer was therefore compared with other commercially available inverse emulsion polymers and thickeners in the presence of these selected electrolytes in an aqueous dispersion at the given pH. The IE-acrylate copolymer demonstrated superior thickening performance (see Figure 3 and Figure 4) and worked as the sole emulsifier, emulsion stabilizer and thickener at pH ≥ 6.5.
Electrolyte Tolerance at pH 5.5–6.5
At pH 5.5–6.5, the IE-acrylate copolymer still offered good performance in low electrolyte-containing systems; however, the addition of a co-thickener such as carbomerc or acrylates/C10–30 alkyl acrylate crosspolymerd, or a coemulsifier was found to further enhance viscosity, electrolyte resistance and aesthetics in the presence of high levels of electrolytes. An emulsion was prepared with 1% w/w solids of the IE-acrylate copolymer, 10% w/w cetyl ethylhexanoate and 0.2% w/w solids acrylates/C10–30 alkyl acrylate crosspolymer in water. At pH 5.5, the emulsion showed enhanced viscosity and stability in the presence of high levels of sodium chloride.
Figure 5 shows that a water dispersion of 1% w/w solids IE-acrylate copolymer in combination with 0.2% w/w acrylates/C10–30 alkyl acrylate crosspolymer provided similar to better electrolyte resistance, compared to 1.2% w/w solids IE-acrylate copolymer, and better aesthetics compared to 1.2% w/w solids acrylates/C10–30 alkyl acrylate crosspolymer in this aqueous dispersion at pH 5.5.
Comparing the viscosities of a combination of the two polymers against those of the IE-acrylate copolymer or the acrylates/C10–30 alkyl acrylate crosspolymer alone, it seems that acrylates/ C10–30 alkyl acrylate crosspolymer could behave synergistically with IE-acrylate copolymer to enhance its electrolyte tolerance and thickening. Such performance may be explained by the fact that microgel size resulting from inverse emulsion polymerization is substantially smaller than that resulting from precipitation polymerization of the acrylates/C10–30 alkyl acrylates crosspolymer. The combination of the polymers of different microgel size can therefore result in a higher packing density, minimizing void space between particles. As both polymers also are hydrophobic, the more efficient packing may help to strengthen interpolymer association, resulting in increased electrolyte tolerance.
Emulsification/co-emulsification: To characterize the stabilization provided by the IE-acrylate copolymer, its ability to emulsify 5%, 10% and 20% w/w of common oils used in skin care systems, such as mineral oil, squalane, caprylic capric triglycerides and isopropyl palmitate, was evaluated. The viscosity provided by 0.4% w/w polymer in o/w emulsions formulated with these oils at dispersion pH was measured. Figure 6 shows that the IE-acrylate copolymer was capable of emulsifying various types of emollients at a low concentration. Further, its ability to impart viscosity to the emulsion increased as the polarity of the oil phase increased.
In addition, the authors studied the effects of IE-acrylate copolymer at 1.0% w/w solids and a co-emulsifier, polysorbate 20, on the viscosity and stability of an emulsion formulated with 10% of the medium polarity oil cetyl ethylhexanoate, comparing it to a control that contained no co-emulsifier. The test and control emulsions were both adjusted to pH 5.5 by the addition of lactic acid. Viscosity was measured with a viscometerb at 25°C, 24 hr after production at room temperature, and the stability was assessed by measuring the emulsion particle size in microscopic pictures taken with a microscope. Table 1 shows that although the IE-acrylate copolymer alone could emulsify the system in the control, the addition of a small amount of co-emulsifier, 0.2% w/w solids, reduced the emulsion droplet size further, and increased the viscosity and stability of the system.
Electrolyte impact in emulsion: To simulate the possible effect of electrolytes in the above emulsion, NaCl, a monovalent salt, was selected as an example source of electrolytes. The electroyte resistance was assessed by adding different concentrations of NaCl in the above test and control emulsions, with and without 0.2% polysorbate-20, and by measuring the viscosity with a viscometerb at 25°C, 24 hr after production at room temperature. As expected, the results showed that in the presence of electrolytes, the addition of a high-HLB co-emulsifier such as polysorbate-20 enhanced the thickening and stabilizing performance of the IE-acrylate copolymer. The addition of polysorbate-20 resulted in additional coverage to the oil-water interface, resulting in smaller oil droplets and an increased total interfacial area, in turn providing higher viscosity than the polymer alone.
In Combination With Actives
Vitamin C derivative for skin lightening: It is common to use inverse emulsion polymers as co-emulsifiers and stabilizers for difficult-to-stabilize emulsion systems, as these polymers reinforce the emulsifying property of the primary emulsifier and act as rheology modifiers to improve the viscosity and stability of the system. The thickening performance of the IEacrylate copolymer was therefore compared against other inverse emulsion polymers in an aqueous dispersion containing various levels, i.e., up to 3.0% w/w solids, of sodium ascorbyl phosphate (SAP), a vitamin C derivative in salt form. This commonly used active is classified in Japan as a quasi drug for skin-whitening formulations, and such a designation requires the minimal use level of 3.0% w/w solids. As reflected in Figure 7, the IE-acrylate copolymer showed better viscosity response than the other polymers in water dispersions containing high levels of the monovalent ascorbic acid salt.
2-Phenylbenzimidazole sulfonic acid (PBSA): To balance skin feel and functionality, many formulators use PBSA, a water-soluble sunscreen with excellent absorption in the UV-B wavelength region, in combination with oil-soluble UV filters. However, PBSA must be neutralized to pH 7–8 prior to use or it may revert to its acid form and crystallize, rendering it less effective as a sunscreen active. At pH 7–8, it is available in its salt form, which will negatively interact with ionic polymers, resulting in low viscosity. Based on results shown in Figure 8, the IE-acrylate copolymer provided the best viscosity in the presence of different levels of neutralized PBSA. Notably, most commercially available inverse emulsion polymers tested were unable to provide viscosity in the presence of just 1% w/w solids PBSA.
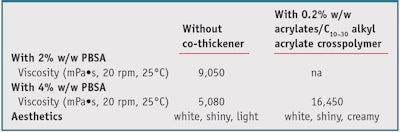
To validate these results in the emulsion system, the authors made a simple cold-process emulsion with 1% w/w polymer solids, 10% cetyl ethylhexanoate and different levels of PBSA, i.e., 2–4% w/w solids, with and without 0.2% w/w solids acrylates/C10–30 alkyl acrylate crosspolymer as a co-thickener. The results in Table 2 illustrate that the IE-acrylate copolymer was able to provide viscosity and stabilization in an emulsion system containing 2% and 4% w/w solids PBSA. Synergy with acrylates/ C10–30 alkyl acrylate crosspolymer enabled the addition of a small amount of this co-thickener to strongly increase the final viscosity of the system containing 4% w/w solids PBSA.
Formulating With IE-acrylate Copolymer
The IE-acrylate copolymer is in liquid form for ease of use. It is versatile, enabling cold or hot processing, and can be added at any stage of the process, even in post-addition. It contains a light carrier oil phase that offers broad flexibility in sensory modification, and has a normal dispersion pH of 6.5–7.5. Since in general, the polarity of an oil impacts emulsion viscosity and stability, it is recommended to use a co-emulsifier or co-thickener with the material in emulsions containing low polarity oils, pH 5.5–6.5, or in the presence of high electrolytic ingredients.
Conclusion
From the data presented, it can be concluded that the IE-acrylate copolymer is an effective inverse emulsion polymer that offers multifunctional benefits. It provides excellent thickening and stabilization properties, especially in systems containing high levels of electrolytes. In addition, it can impart efficient synergistic thickening with carbomer or acrylates/C10–30 alkyl acrylate crosspolymer, which enables further enhanced performance, especially under challenging conditions. Its ease-of-use and its improved performance compared to existing inverse emulsion technologies make it a novel solution to offer viscosity and stabilization to any challenging system.
Disclaimer: The information contained herein is believed to be reliable, but no representations, guarantees or warranties of any kind are made as to its accuracy, suitability for a particular application or the results to be obtained herefrom. Lubrizol Advanced Materials, Inc. (“Lubrizol”) cannot guarantee how any products associated with this information will perform in combination with other substances or in your process as the “user.” Often, the information is based on laboratory work with small-scale equipment. Due to variations in methods, conditions and equipment used commercially in processing materials, the information does not necessarily indicate end product performance or reproducibility. As such, no warranties or guarantees are made as to the suitability of the information or products referenced hereunder for any applications disclosed to Lubrizol. Full scale testing and end product performance are the responsibility of the user. Further, any formulations presented hereunder should be used only as a suggested starting point. Lubrizol shall not be liable and the user assumes all risk and responsibility for, any use or handling of any material beyond Lubrizol’s direct control. The seller makes no warranties, express or implied, including, but not limited to, the implied warranties of merchantability or fitness for a particular purpose. Nothing contained herein is to be considered as a permission, recommendation or as an inducement to practice any patented invention without permission of the patent owner.
References
Send e-mail to [email protected].
- AV Rawling, D Canestrani and B Dobkowski, Moisturizing technology versus clinical performance, Dermatological Therapy 17: 49–56 (2004)
- A Chudasama, V Patel, M Nivsarkar, K Vasu and C Shishoo, Investigation of microemulsion system for transdermal delivery of itraconazole, J Adv Pharma Tech and Res 2(1) 30–38 (2011)
- R Lochhead, Trends in polymers for skin care, Part I, Happi 4: 83–87 (2009)
- S Herman, Hitting the sauce, DCI 18–20 (Oct 1998)
- T Tadros, Application of rheology for assessment and prediction of the long-term physical stability of emulsions, Advances in Colloids and Interface Science 108–109, 227–258 (2004)
- RY Lochhead, Electrosteric stabilization of oil-in-water emulsions by hydrophobically modified poly(acrylic acid) thickeners, ACS Advances in Chemistry Series 462, ch 6 (1991) pp 101–120