The majority of cleansing products on the market contain surfactants that function to remove dirt, excess sebum or other residues from the skin.1 However, after repeated use, surfactants can lead to dryness and trigger further skin conditions. This is because, along with dirt and residues, high concentrations of surfactants also remove or damage skin’s lamellar structures;2 although the mechanism by which this occurs is not fully understood. These lamellar lipid structures, including cholesterol, ceramides and fatty acids, surround the corneocytes of the stratum corneum (SC) and play a significant role in regulating water transport through the SC by contributing to the skin’s brick and mortar structure.
Studies suggest that surfactants solubilize these skin lipids into micelles, resulting in dissolution of the SC lipids. They also suggest that surfactant incorporation into the bilayer causes instability and greater permeability, leading to water loss.3, 4 In addition, surfactants remove the skin’s Natural Moisturizing Factor (NMF), an intrinsic humectant consisting of hygroscopic components such as urea and amino acids. These components are soluble in water, and their removal results in skin dryness.5, 6 Surfactant exposure has a negative effect on certain components of NMF as well, such as amino acids derived from the degradation of filaggrin, which are required for the generation of skin cells.5 Furthermore, surfactants can gradually increase skin pH due to their alkaline nature, which can be detrimental to the SC; studies suggest a lower pH is preferable for SC integrity and skin barrier homeostasis.7
To increase the mildness of cleansing formulations, combinations of surfactants have been utilized. For example, the anionic surfactant sodium laureth sulfate often is used with the amphoteric surfactant cocamidopropyl betaine to minimize skin irritation potential.4 However, there remains a need for moisturizing cleansing systems. Moreover, busy consumer lifestyles have increasingly fuelled the trend for products that provide convenient and time-saving solutions. This also has increased the demand for delivering moisturization through wash-off products. In 2013, nearly 40% of all hand and body washes made a moisturization/hydration claim.1 Although the two basic requirements for wash-off moisturization products are simple, the answer is not. For a product to really deliver, it must physically hydrate the skin and provide benefits that the consumer can see and feel.
In response, studies were undertaken to determine whether the deposition of a moisturizing ingredient from a cleansing system could reduce surfactant-induced damage. An optimized moisturization complexa was developed for inclusion in the surfactant studies. Each component within the complex served a function in moisturizing skin, including improving the bilayer structure, replenishing fatty acids, and optimizing the packing parameter of the formulation lipids. Regarding the latter, the more tightly packed the lipids are in the formulation, the more difficult it is for water to leave the skin upon application.
This oil-based complex was also insoluble in water, increasing the likelihood of it being deposited onto skin during cleansing to provide a moisturization benefit as well as perceived sensory benefits. Changes in skin protein content, visible skin dryness, transepidermal water loss (TEWL), corneometry and sensorial characteristics were then measured and analyzed.
Materials and Methods
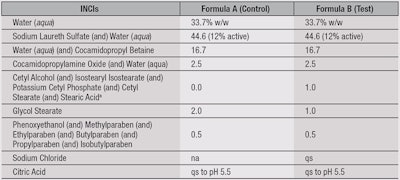
Clinical studies, instrumental: Fifteen healthy Caucasian panelists, ages 21–42, were recruited for a clinical study. The protocol consisted of a five-day wash-out phase with a commercial soap, followed by a 14-day treatment phase with formulations A and B, in a randomized and blind manner, on either volar forearm (see Table 1). During both the wash-out and treatment phases, panelists were instructed to wash their volar forearms three times daily, and instructed to not use other products or moisturizers on the treatment area.
Instrumental measurements were taken on days 0 (baseline), 2, 4, 8, 10 and 14 at a temperature and humidity controlled facility, 50% RH ± 5.0 and 20°C ± 1.0, with an acclimation period of 20 min prior to testing. Parameters measured included TEWLb, corneometryc and protein content, which was collected using sampling discsd to remove a uniform sample of SC from the volar forearm. A detection devicee was then used to indirectly measure the SC protein content on the sampling discs by measuring the optical absorption at 850 nm. This value was translated into protein content using the formula: y = a • x – b. Finally, a digital microscope (20 × magnification) was used to capture images.
Statistical analysis: Data was collected and subjected to the Grubbs Outlier tests to ensure all data was within a normal distribution, followed by parametric paired t-tests. A non-parametric Wilcoxon Signed-Ranks Test was used for analysis of the sensory data. Statistical significance was set at 95% for both tests.
Clinical studies, sensory: In a separate clinical study, 30 healthy Caucasian panelists ages 21–44 were recruited and instructed to wash their volar forearms with a commercial body wash using a specified washing and drying protocol. This was followed by a questionnaire to rate the levels of softness, moisturization and tightness of their skin on a scale from 1–10. The protocol was then repeated with 0.2 mL of test formulations A and B (see Table 1) on the same areas of the forearms followed again by the questionnaire to assess any differences in sensory characteristics imparted to the skin.
X-ray diffraction: Wide angle x-ray diffraction (WAXD) and small angle x-ray diffraction (SAXD) were also performed on the test formulations and compared with a control formulation to aid in understanding how the formulations improved skin condition. Formulations were dried for 1 hr at 32°C, allowing a representative lipid structure to form as it would on skin after application. The methodology and formulations used for this study are described by Pennick et al.8 Essentially, WAXD was used to examine the packing of lipids, while SAXD was performed to characterize effects on sensory parameters.
Results and Discussion: Instrumental Assessments
For the duration of the study, the net changes in protein content, TEWL and corneometry were calculated from day 0 (baseline) to analyze the effects of both formulations on the skin.
Protein content: Skin protein content, i.e., the number of corneocytes removed from the skin, increased for both formulations (see Figure 1), which indicates reduced corneocyte adhesion within the SC. As previous studies have shown, this is due to the decomposition of the SC into individual corneocytes. Thus, on an exaggerated wash study with high surfactant exposure, an increase in protein content was expected. This decrease in cohesion of the cells could be due to surfactants removing the lipids, hence the corneocytes become more loosely attached and the cells are more easily lost.3, 9, 10
However, the increase in skin protein content was lower for test formulation B—particularly on day 14 at p < 0.05, which showed fewer corneocytes on the sampling disc. This suggests the test product improved the adhesion of corneocytes in the SC in comparison with the control, also indicating a milder formulation.
These protein content results also show that surfactant-induced damage can lead to an increase in skin dryness. To determine whether this could be visually perceived, and whether the moisturization complex could lessen the visible damage, images were taken of the treated sites (see Figure 2). On day 0, the images show the skin in good condition. By day 14 of the treatment phase, images for both formulations A and B show skin damage, which appears as white flaking, indicating dry skin. However, formulation B had less visible dryness, correlating well with the protein content results and suggesting that the formulation is milder to the skin and less drying.
TEWL: As well as analyzing the skin’s structural condition, TEWL was measured to determine whether the moisturization complex improved skin moisture. Since the protocol involved repetitive cleansing of the skin without the use of other body products, an increase in TEWL was expected for both formulations. As noted previously, this could theoretically be attributed to lipids surrounding the corneocytes being damaged or lost, providing a more penetrable path for water to leave the skin. In addition, residual surfactant could remain on the skin after rinsing, inserting itself into the lipid lamellae and disrupting the SC, increasing TEWL.2, 4, 11
The TEWL of formulation B increased less than formulation A, significantly after day eight (p < 0.05), indicating formulation B had a moisturizing effect on the skin (see Figure 3). This improvement in TEWL, compared with the control, occurred as the moisturization complex improved the packing parameter of the formulation lipids and provided the fatty acids skin required to maintain a healthy barrier.
WAXD: To provide evidence for this moisturizing mechanism, WAXD was performed on the moisturization complex in a leave-on emulsion. Results can be seen in Figure 4, which provides information about the lateral packing of the lipids in the lamellar phase of the formulation. In the control, the distances between the lipid head groups were 0.47 nm and 0.40 nm, whereas the distances between the head groups in the moisturization complex were 0.42 nm and 0.38 nm, meaning the packing was much tighter, i.e., orthorhombic lateral packing. Since the packing parameter of the lipid head groups determines permeability, a tightly packed structure has a lower TEWL. This provides an understanding as to why the TEWL of formulation B was lower than that of A; the moisturization complex stabilized the orthorhombic packing of the formulation lipids.
Corneometry: Skin water content was analyzed to determine whether the use of a moisturizing ingredient in a surfactant system could lead to increased hydration in the skin (see Figure 5). Results indicated the water content of skin decreased upon repeated application with formulation A, whereas formulation B slightly increased water content. By day four of the study, the water content of skin with formulation B was significantly higher than with formulation A (p < 0.05), and after day 10 this increased water content was significant at p < 0.01.
A decrease in the corneometry value of skin exposed to surfactants could be due to the disruption of the corneocyte structure. Capacitive pixel-sensing technology studies have shown that surfactants, particularly anionic surfactants such as sodium laureth sulfate, bind to corneocytes and induce swelling, leading to the loss of corneocyte hydration.12 Additionally, removal of NMF from skin during cleansing can lead to a lower capacitance reading since there is less secondary bound water in the skin’s corneocytes.4 The corneometry values shown here indicate not only that the moisturization complex reduced the damaged caused by the surfactants, but also produced a slight increase in skin hydration.
Results and Discussion: Sensory Assessments
Thus far, the instrumental data has shown an increase in skin moisturization and improvement in skin structure. However, it is also important for the surfactant-based formulation to impart a positive sensory experience to the skin. The sensorial study therefore analyzed any changes in the perception of skin softness, moisturization and tightness both before and after washing with test formulations A and B.
As shown in Figure 6, a highly significant increase (p < 0.01) in skin moisturization was observed by panelists after washing with formulation B; with formulation A, this increase was insignificant. For skin softness, a statistical improvement was obtained with formulation B (p < 0.05), whereas the increase was, again, insignificant for formulation A. Due to this increase in perceived softness and moisturization, it was highly likely that the moisturization complex was effectively depositing on the skin. Further internal data was therefore generated to research this deposition.
Within a 30-min period, a six-time repeated wash study using a surfactant-based system was conducted to determine whether the moisturization complex had instant effects. Results indicated the increase in TEWL for the moisturization complex was significantly lower than for the control, even with the increased exposure, therefore strongly suggesting deposition onto the skin since the barrier showed instant improvement in water loss (data not shown).
SAXD: To aid in understanding how the positive sensorial feel was obtained from the moisturization complex, SAXD was performed on it in a leave-on emulsion. This analysis provides information regarding periodicity within stacks of skin bilayers,13 which would indicate whether the formulation caused an increase in the thickness of the layers formed and thus influenced sensory characteristics. Results from the SAXD showed the control formulation created lipid bilayers 5.0 nm thick, while the test formulation created lipid bilayers twice as thick: 10.0 nm, as shown by the peaks in Figure 7. This suggests the moisturization complex would provide the same effect in a surfactant system. The thicker lipid bilayers assist with the observed sensory characteristics. In addition, since the sensory characteristics improved with single use of the product, as the data indicates, the moisturization complex is effectively depositing onto skin from the surfactant system.
Conclusion
The instrumental and sensorial studies described here demonstrate that the addition of a moisturization complex at 1% in a surfactant system could physically improve skin moisturization and structural condition, as well as provide a consumer-perceivable sensorial benefit, when compared with a control formulation. In addition to further internally generated data, these results strongly indicate the successful deposition of the moisturization complex onto skin from a surfactant-based system, which is one of the most difficult hurdles to overcome in the cleansing market. This advance enables the development of multi-functional cleansing and moisturizing ingredients to meet current market demands.
References
- www.gnpd.com/sinatra/gnpd/frontpage/ (Accessed Jul 22, 2014) (subscription required)
- R Walters, G Mao, E Gunn and S Hornby, Cleansing formulations that respect skin barrier integrity, Dermatology Research and Practice 1–9 (2012)
- S-W Lee, K Tettey, Y Yarovoy and D Lee, Effects of anionic surfactants on the water permeability of a model stratum corneum lipid membrane, Langmuir 30(1) 220–226 (2013)
- J Levin and R Miller, A guide to the ingredients and potential benefits of over the counter cleansers and moisturizers for rosacea patients, J Clinical and Aesthetic Derm 4(8) 31–49 (2011)
- DR Hoffman, LM Kroll, A Basehoar, B Reece, CT Cunningham and DW Koenig, Immediate and extended effects of sodium lauryl sulfate exposure on stratum corneum natural moisturizing factor, Intl J Cos Sci 36 93–101 (2014)
- C Harding, J Bartolone and AV Rawlings, Effects of natural moisturizing factor and lactic acid isomers on skin function, in Dry Skin and Moisturizers: Chemistry and Function, M Loden and H Maibach, eds, CRC Press, Boca Raton, FL USA 187–210 (2004)
- JW Fluhr et al, Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity, J Invest Derm 117 52–58 (2001)
- G Pennick, B Chavan, B Summers and AV Rawlings, The effect of an amphiphilic self-assembled lipid lamellar phase on the relief of dry skin, Intl J Cos Sci 34 567–574 (2012)
- GJ Putterman, NF Wolejsza, MA Wolfram and K Laden, The effect of detergents on swelling of stratum corneum, J Soc Cos Chem 28 521–532 (1977)
- LD Rhein, CR Robbins, K Fernee and R Cantore, Surfactant structure effects on swelling of isolated human stratum corneum, J Soc Cos Chem 37 125–139 (1986)
- S Ghosh, S Hornby, G Grove, C Zerwick, Y Appa and D Blankschtein, Ranking of aqueous surfactant-humectant systems based on an analysis of in vitro and in vivo skin barrier perturbation measurements, J Cos Sci 58(6) 599–620 (2007)
- E Uhoda, JL Lévêque and GE Piérard, Silicon image sensor technology for in vivo detection of surfactant-induced corneocyte swelling and drying, Dermatology 210(3) 184–188 (2005)
- JA Bouwstra and M Ponec, Review: The skin barrier in healthy and diseased state, Biochimica at Biophysica Acta 2080–2095 (2006)