Proteus, in Greek mythology, is ascribed the god of “elusive sea change,” which suggests the constantly changing nature of sea water. Indeed, this god is said to change shape to avoid interrogation; he will answer only to those capable of capturing him. From this character comes the adjective protean, meaning versatile, mutable and capable of assuming many forms.1 It carries the positive connotations of flexibility, versatility and adaptability.
This term also can apply to cosmetic ingredients that display flexible behavior and assume many roles in formulas according to temperature, pH, concentration, etc., and it is common to use one ingredient to perform several formula requirements. Some notable stars in the universe of cosmetic raw materials exhibit an astonishing number of multi-tasking behaviors, examples of which are provided here.
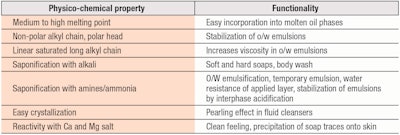
Soap, Structure and Sensory: Stearic Acid Adaptations
To start, one pillar of cosmetic recipes is stearic acid. This molecule, as a component of animal fats, gave rise to the development of the first surfactant, soap, which of course is still used today. In prehistoric times, drops of animal fats produced while meats were roasting over fire came into contact with hot alkaline ashes.2 At this high temperature, they formed soap from the potassium salts of superior fatty acids, among them, stearic. Primitive man quickly realized the foaming and cleansing properties of such a compound.
Cleansing soap: Modern man learned that stoichiometric amounts of alkali give cleansing soaps that perform well. Furthermore, when used at reduced proportions of alkali, so-called neutral or super-fatted soaps were created, providing better skin compatibility and a lower impact on skin pH. Moreover, soaps of different hardness can be made by switching the alkaline neutralizer from sodium to potassium. This is particularly useful when formulating soft shaving creams.
Emulsifier: The big discovery of stearic acid’s versatility, however, took place with the birth of triethanolamine stearate, made possible by the invention of alkanolamines at the beginning of last century. This soap found immediate application as an emulsifier, since its HLB value is around 12 and ideal for nicely emulsifying mineral oils3 and related molecules. In this way, the era of light emulsions began.
The success of this system continues even today, as triethanolamine stearate makes a special type of temporary or transient emulsifier. When emulsions based on it are applied to the skin, the physiological acids of the superficial skin layer react with the soap, resulting in an insoluble stearic acid that finely precipitates over the skin surface and loses its emulsifying properties. This layer exhibits good water and wear resistance, and is frequently applied in foundations and sunscreen formulas to provide skin adhesion and water resistance.
The relatively weak acidity of stearic acid4 also is demonstrated in emulsion mascaras. Mascaras are challenging products. They require waxes that must be emulsified to ensure product stability and easy application to the lashes, yet at the same time, good water-resistance after application. Therefore, if stearic acid is neutralized with ammonia during the emulsification phase, the ammonium stearate emulsifier, applied onto the lashes, will slowly disappear following its reaction with atmospheric CO2 to give ammonium carbonate. Again, insoluble stearic acid is then formed, which prevents the re-emulsification of the pigmented wax layer by moisture.
Structuring agent: Stearic acid also is a structuring agent in emulsions. It increases the viscosity of the oil phase in o/w emulsions but its overall effects on emulsion viscosity are limited. More importantly, it can stabilize oil droplets in the same way cetyl alcohol does; its polar head floats over the surface of emulsified oil droplets while its rigid hydrocarbon chain maintains droplet shapes and dimensions.
In fact, a smart system could be developed to create such a superficial arrangement of stearic molecules around oil droplets. A careful partial precipitation from its alkaline soaps can be performed by adding a small amount of citric or lactic acid at the end of the production cycle. While soap created at the moment of emulsification helps to obtain very subdivided droplets, this late addition of acid will subtract the alkali counter-ion surrounding the emulsified droplets. Thus, stearic acid will precipitate exactly at the interphase level as required and form a solid, well-ordered, spherical layer around each individual droplet.5
In spite of the wide use of stearate soaps as emulsifiers, stearate soaps are not generally added as such to formulas. For ease of handling, the solid stearic acid is incorporated into the oil and fat blend and saponified at a high temperature with alkaline materials previously dissolved in the water phase. This method has drawbacks. One is that the reaction takes place between heterogeneous phases, i.e., between the alkali in water and the acid dissolved into the oil droplets. Therefore, the speed of reaction is strongly dependent on temperature, mixing time and alkali concentration.6
Variable saponification degrees give rise to wayward fluidity of the resulting emulsion due to the changing ratio between non-neutralized acid and neutralized stearate. A general suggestion would be to carry out this step in different batches, always at the same temperature, with the maximum possible concentration of alkali in water, and for a fixed time. After completing the reaction, the blend is then diluted with the proper amount of water.
Sensorial behavior: An interesting side benefit of stearic soaps in cleansers is their sensorial behavior. After cleansing with the soap, during rinsing, the complete elimination of the surfactant is perceived as braking as the fingers slide over the skin. This is generally due calcium and magnesium ions in the tap water forming soaps with the residual alkaline stearate on skin. Such a skin feel makes the sensory difference between synthetic surfactants and soaps.
There are other ways to obtain this same “you are done, stop rinsing” signal. In Asia, many body washes are made by a blend of traditional synthetic surfactants, such as lauryl ether sulfates and the like, which form stearic soaps in situ during batch production. This is a smart approach, even if large amounts of foam are formed when sodium hydroxide, which contains carbonates, is added. Carbon dioxide is released when the reaction of stearic acid takes place and forms stable and abundant foams in the main mixer. This might be one case where the addition of previously prepared soaps could solve the problem.
Another sensory characteristic of stearic acid is its pearling effect7 in combination with glycol stearates in shampoo. The crystallinity of stearic acid as it separates due to temperature decreases provides a frost effect, which further develops as the blend cools. Table 1 summarizes this and the other roles of stearic acid described above.
Betaine Balance, Buffering and Beyond
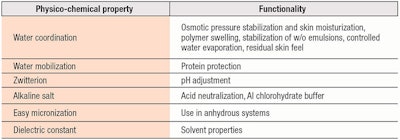
The simplest amphoteric molecule, betaine, is another good example of a substance with multi-tasking cosmetic effects. Its physiological role in nature is the osmotic protection of living cells. In other words, it helps to balance and compensate for changes in osmotic pressure due to different concentrations of solutes between the inside of the cell membrane and its environment. Its physiological activity helps to maintain the equilibrium of skin cells as moisture conditions in their environment change. At the same time, betaine protects the backbone of cell proteins from hydrolysis, preventing their denaturation. These are good principles for achieving skin moisturization.
Formulating aid, stabilizer and thickener: In cosmetic formulas, betaine helps to maintain the water content of emulsions, protecting them from evaporation.8 Moreover, if dissolved in the aqueous phase of w/o emulsions, betaine has a long-term stabilizing effect. Additionally, it can make the production process faster. When dissolved in water, betaine helps to speed the swelling of hydrophilic thickeners, e.g., carbomers and hydroxyethyl cellulose, and achieves superior levels of viscosity, even when used at a fairly low concentration (1-2%).
Neutralizer, buffer and solubilizer: At higher concentrations, betaine can function as a non-traditional neutralizer of carbomers. This is due to the alkaline side of the molecule being a stronger basis than its carboxylic moiety. For the same reason, it has a good buffering effect toward the addition of strong acids to a formula. Moreover, such a buffering effect is capable of adjusting the formulae of antiperspirants containing aluminum chlorohydrate to more moderate, non-aggressive pH values. Another performance of betaine, due to its special modification of the organization of water molecules, is its ability to help dissolve sparingly soluble substances, e.g., allantoin and salicylic acid, in water even at cold temperatures.
Water-soluble or milled for anhydrous application: All of the described properties of betaine are accomplished in aqueous solutions, as it is a very water-soluble molecule. Beyond the water dominion, however, betaine is insoluble. To overcome this drawback, it has been micronized, i.e., milled to very fine particle size, which enables its inclusion in anhydrous makeup products. Formulation experiments9 show this form to impart superior application properties in lipsticks, foundations, anhydrous mascara and compact eye shadow, in terms of ease of application over the skin, lips and lashes, and softness.
Light touch: Betaine also has a nice sensory profile. When blended with glycerin and/or propylene glycol in aqueous phases, it changes the final touch of an emulsion, giving the perception of quicker absorption and a light feel. All this at low amounts, generally from 1% to 2%. Table 2 summarizes this and the other roles of betaine described above.
Titanium Dioxide, Color Chameleon
Titanium dioxide is another “pearl” for the cosmetic formulator. In association with silicates and metallic oxides, the pigmented pearls obtained from it are and largely used in makeup. By changing the thickness of titanium dioxide layers deposited onto silicate platelets and sandwiching these layers with oxides, silica and organic pigments, a rainbow of metallic, shiny and pearlescent effects can be obtained. Many makeup products are built based on these iridescent effects.
In its physical, crystalline rutile or anatase form, the small particles of titanium dioxide impart whitening effects to both formulas and skin. In makeup, this provides light reflection and luminosity to the final color. It is said to “develop the color” by increasing the visible lightness and showing the many nuance possibilities of all other pigments. On the other hand, it gives opacity to the product layer applied to the skin, providing a hiding effect against blemishes and skin imperfection. In other product categories, it hides yellowish formula discoloration that can occur over a product’s shelf-life.
Finally, in small dimensions, down to the nano-scale and coated with stabilizing ingredients,10 it imparts high transparency to visible light and becomes an efficient UV filter, suitable for all types of sunscreens. Indeed, titanium dioxide is a perfect example of the potential to change the properties of a solid ingredient by modifying its dimensions and conditions of use.
Xanthan-tastic Dynamics
Many other examples would be worth mentioning, and probably each formulator has a preferred protean ingredient. I personally like xanthan gum, whose effects adapt according to its concentration.11 At high levels of 0.2-0.5%, it thickens; note that higher quantities become too sticky. At low concentrations of 0.2-0.3%, it is less effective as a thickener but becomes a good suspending agent. At even lower concentrations, i.e., 0.05--0.15%, it works as an emulsion stabilizer by providing elasticity to the emulsion network.
Forces of Fragrance
Let’s conclude this list with a special category of sensory ingredients—perfumes. One might think their only function is sensorial, i.e., to mask off odors and provide an appealing scent in the end product. Indeed, the truth is much broader. Perfumes are frequently used to dissolve insoluble ingredients throughout the course of the production process, which leverages their fluid form and very high solvent power.
Moreover, fragrances can reduce the viscosity of the intermediate phase of the production run in concentrated cleansers. Additionally, they can be used to control the wetting and swelling speed of hydrophilic polymers. For instance, by adding perfume in its aroma form to carboxymethyl cellulose, which is used in many toothpastes, a hydrophobic layer is formed over the surface of the abrasive granules. The addition of such a wet powder into the mixer prevents the formation of large lumps and better controls the polymer swelling in water.
Conclusions
Considering cosmetic ingredients and their less evident properties, the diversity of possible behaviors is astonishing. Their chemical structures, physical forms, addition methods, interactions with other ingredients, etc., create a fascinating never-ending tale of cosmetic formulation.
References
- merriam-webster.com/dictionary/protean (Accessed Sep 17, 2016)
- soaphistory.net/soap-history/who-invented-soap/ (Accessed Sep 20, 2016)
- M Rieger and LD Rhein, Surfactants in Cosmetics in Surfactant Science Series, 2nd edn, CRC Press, Boca Raton, FL USA (68)(1997) p 129
- https://en.wikipedia.org/wiki/Sodium_stearate (Accessed Sept 20, 2016)
- EP Becher, Encyclopedia of Emulsion Technology, vol 2, Applications, Marcel Dekker, New York (1985) p 85
- FD Gunstone, JL Harwood and FB Padley, The Lipid Handbook, 2nd edn, CRC Press, Boca Raton, FL USA 306-8 (1994)
- google.com/patents/EP0215025A1?cl=en (Accessed Sep 28, 2016)
- L Rigano, G Dell’Acqua and R Leporatti, Benefits of trimethylglycine (betaine) in personal care formulations, Cosm & Toil (May 2003)
- in-cosmetics.com/__novadocuments/7930 (Accessed Oct 14, 2016)
- JF Jacobs, I van de Poel and P Osseweijer, Sunscreens with titanium dioxide (TiO2) nano-particles: A societal experiment, Nanoethics 4(2) 103-113 (2010)
- DFS Petri, Xanthan gum: A versatile biopolymer for biomedical and technological applications, J Appl Polym Sci 132, 42035 (2015)