Retinoids are a class of chemical compounds that are similar in structure to or derived from vitamin A. Retinoids serve many important and diverse functions throughout the body, including roles in vision,1 regulation of cell proliferation and differentiation,2 growth of bone tissue,3, 4 immune functions,5 and as activators of tumor suppressor genes.6 These compounds are also being investigated as preventive agents for skin cancer.7
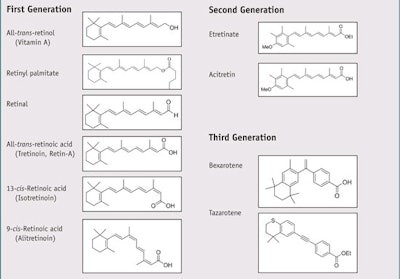
The basic structure of a retinoid compound consists of four isoprenoid units joined head-to-tail with a cyclic group on one end, a polyene side chain, and a functional group on the other end.8 The conjugation of the polyene side chain—i.e. alternating single and double bonds—is responsible for the color of retinoids, which are typically yellow, orange or red, as well as their ability to act as chromophores. Variations in the side chains and polar end groups of these compounds lead to different classes of retinoids. Retinoids are classified into three generations (see Table 1),9 the first of which contains naturally occurring retinoids, though all are also produced synthetically. Both first- and second-generation retinoids are ligands for (bind to) several retinoid-binding proteins, called receptors, found in serum, cytoplasm and nuclei. Third- generation retinoids are less flexible than first- and second-generation retinoids and interact with fewer retinoid receptors. The body stores the majority of its retinoid reserves in the liver, mostly as retinyl esters of palmitic, stearic, oleic and linoleic acids.10
Once retinol, or vitamin A, has been taken up by a cell, it can be oxidized to retinal, which is further oxidized to retinoic acid.11 Retinoic acid acts as a ligand for both the RAR and retinoid X receptors (RXR). Retinol appears to function in maintaining skin health and is being used in an increasing number of consumer skin care products for treating aged skin and wrinkles, presumably by stimulating new collagen production.
Retinyl palmitate is the ester of vitamin A and the linear saturated C-16 fatty acid known as palmitic acid. This retinoid is the one most often found in cosmetic products including sunscreens. While retinyl palmitate is more thermally stable than retinol,12 it is still thermally unstable. For instance, one study of an o/w emulsion containing 2% retinyl palmitate stored at 4°C showed a 38% loss of retinyl palmitate after seven days.12 In regard to photostability, retinyl palmitate has been shown to decompose even faster than retinol when exposed to UVA radiation (320–400 nm).12 Further, when topically applied, retinyl palmitate has been shown to penetrate the skin, where it is hydrolyzed to retinol by esterases contained therein.13
First-generation retinoids are notoriously unstable in the presence of heat, oxygen and light.14 Photo-induced chemical reactions produce various retinoid isomers, e.g. trans to cis;15 oxidized products such as epoxides; and photodecomposition products including anhydrous retinoids and non-retinoids. 16–21 The photo-irradiation of retinyl palmitate in the presence of a lipid has been shown to induce lipid peroxidation and free radical formation.22
Considering these instability issues, the authors sought to determine whether ethylhexyl methoxycrylene (EHMC), an effective photostabilizer for certain organic UV filters (see Figure 1), could perform the same photostabilizing function for topically applied retinoids. This material has previously been shown to operate via a singlet-quenching mechanism in sunscreens.23
Materials and Methods
Materials: For the present study, retinol (99%) and retinyl palmitate (18,000,000 USP Units/G) were obtaineda while the authors’ company supplied the EHMCb. All other ingredients for the test formulations were acquired as samples from well-known cosmetic raw material suppliers.
UV/visual spectra: The ultraviolet and visual (UV/vis) absorbance spectra of retinol and retinyl palmitate were determined by spectrophotometerc (see Figure 2). The spectra for retinol were determined at 10 ppm in methanol, whereas due to its poor solubility in methanol, the spectra for retinyl palmitate were determined at 10 ppm in tetrahydrofuran.
Fluorescence quenching: Qualitative fluorescence quenching experiments were performed by preparing dilute solutions (0.2% w/w) of retinol in ethyl acetate. Three solutions contained retinol alone or combined with 1% and 2% of a non-photoactive diluent, i.e. phenethyl benzoate, which served as negative control solutions. Two additional solutions were prepared with 1% and 2% EHMC in place of a portion of the non-photoactive diluent, which served as the test solutions.
Fifteen microliters each of the control and test solutions were spotted on thin layer chromatography (TLC) platesd using a micropipette and allowed to dry. The TLC plates were then illuminated with long-wave UV radiatione and a photographf of the illuminated plate was taken (see Figure 3).
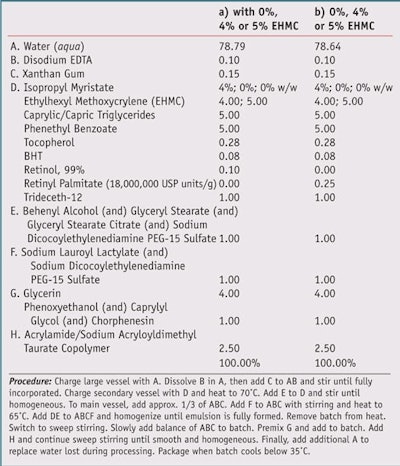
Test formulations: To test the effects of simulated solar UV radiation on basic products containing retinol, three formulations were prepared, each containing 0.1% retinol; one contained no photostabilizer, one contained 4% EHMC, and one contained 5% EHMC. Similarly, three formulations containing 0.25% retinyl palmitate, alltrans- retinol ester of palmitic acid, were prepared as traditional o/w emulsions (see Table 2).
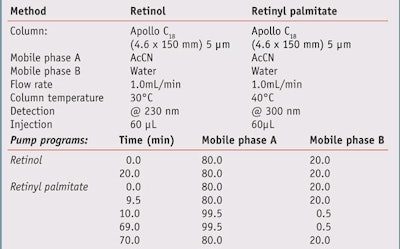
High-performance liquid chromatography (HPLC) studies: A sample of each test formula was analyzed by HPLC for retinol or retinyl palmitate content before and after irradiation with simulated solar UV radiation. Chromatographic analyses were performed on a systemg equipped with quaternary pumps, a vacuum degasser, an auto-injector and a dynamic absorbance detector (DAD), all connected to a computer running specialized softwareh. The reagents, acetonitrile (AcCN) and water, were both HPLC grade. Chromatographic separation was achieved using a C18 columnj under the conditions summarized in Table 3.
The samples for analysis by HPLC were prepared in the dark. Approximately 0.12 g of a test formulation was applied to a 5 cm x 2.5 cm quartz slide with each dose covering a 1-cm circle. Irradiated slides were exposed to 5 minimum erythemal doses (MED) of full spectrum UVR (290–400 nm) at three different locations. While 1 MED = 21 mJ/cm2, 5 MED is roughly equivalent to 1 hr of midday, midsummer sun exposure.
Following irradiation, residual sample material was extracted from the slides with AcCN and the resulting mixtures were mixed well and filtered through PTFE filters before being subjected to HPLC analysis. Non-irradiated samples were processed in an identical manner. Butylatedhydroxytoluene (BHT), present at 0.08% of the formulation, was used as the internal standard for calculating retinol content, whereas the standard for calculating retinyl palmitate was α-tocopherol, present at 0.28% of the formulation.
A solar simulator equipped with a UVB detector and microprocessorbased controllerk provided the UV radiation. The xenon lamp’s output in the solar simulator was filtered to remove wavelengths < 290 nm, i.e. UVC, and > 400 nm, i.e. visible light and infrared radiation.
UV transmittance analysis: The formulations in Table 2 with either 0% EHMC or 4% EHMC were also subjected to a UV challenge and measured for photostability on a transmittance analyzerm. UV radiation was provided by the same solar simulatork. Test samples were prepared by applying and spreading 32.5 μL of the formulations evenly on a 5 cm x 5 cm polymethyl methacrylate (PMMA) platen using a micropipette. The plate was dried in the dark for 15–20 min, after which the center of the plate was scanned on the UV transmittance analyzer. The center of the plate was then irradiated with 5 MED full spectrum UV radiation, i.e. 290–400 nm, and scanned again. The resulting absorbance spectra were normalized and overlaid for comparison.
Results
UV spectra: Retinol absorbed UVR with a peak at 325 nm and a molar extinction coefficient (ε) of 53,500. Retinyl palmitate absorbed UVR with a peak at 329 nm and a molar extinction coefficient (ε) of 45,500. The absorbance spectra of both retinoids is shown in Figure 2.
Fluorescence quenching: Under long-wave UV radiation, the solution of 0.2% retinol in ethyl acetate exhibited a strong visible fluorescence, indicating the emission of a photon from the singlet excited state. As shown in Figure 3, this fluorescence is hardly visible in the spot with the inclusion of 1% (w/w) EHMC, and not at all visible in the spot with 2% EHMC. This indicates that in the presence of EHMC, retinol’s fluorescence is more or less completely quenched. From this qualitative finding, it is reasonable to assume that EHMC operates on the singlet excited state of retinol just as it does on certain UV filters.
HPLC studies, 0.1% retinol: For the formulations containing 0.1% retinol, BHT, the internal standard, eluted between 7.71 and 8.02 min. Retinol eluted between 12.56 and 13.46 min. The E and Z isomers of EHMC eluted at 9.13 and 10.27 min, respectively. For the formulation without EHMC, the ratio of the heights of the BHT and retinol peaks before irradiation was 3.27; after 5 MED, the ratio was 15.36, indicating a loss of 79% of the retinol.
For the formulation with 4% EHMC, the ratio of the two peaks was 3.40 before irradiation and after 5 MED, the ratio was 3.57, indicating a loss of less than 5% of the retinol. Finally, for the formulation with 5% EHMC, the ratio of the two peaks was 3.48 before irradiation and after 5 MED, the ratio was 3.50, indicating virtually no loss of retinol. The results of the HPLC studies of the three formulations containing 0.1% retinol are summarized graphically in Figure 4, and portions of the chromatograms of two of the irradiated samples appear in Figures 5a and b.
HPLC studies, 0.25 % retinyl palmitate: For the formulations containing 0.25% retinyl palmitate, tocopherol, the internal standard, eluted at 23.24 to 23.56 min. Retinyl palmitate eluted at 50.85 to 51.64 min and the E and Z isomers of EHMC eluted at 8.21 and 9.24 min, respectively. For the formulation without EHMC, the ratio of the areas of the peaks for tocopherol and retinyl palmitate was 0.179 before irradiation, and 0.477 after 5 MED, indicating a 62% loss of retinyl palmitate.
For the formulation with 4% EHMC, the ratio of the two peaks was 0.189 before irradiation and 0.198 after 5 MED, indicating a loss of retinyl palmitate of less than 5%. For the formulation with 5% EHMC, the ratio of the two peaks was 0.178 before irradiation and 0.179 after 5 MED, indicating no loss of retinyl palmitate. The results of the HPLC studies of the three formulations containing 0.25% retinyl palmitate are summarized graphically in Figure 4 and the chromatograms of two irradiated samples are shown in Figures 6a and b.
UV transmittance: In the formulations without EHMC, irradiation with 5 MED caused the peak UV absorbances of retinol at 325 nm and retinyl palmitate at 329 nm to decline by 86% and 93%, respectively. In the formulations with 4% EHMC, irradiation caused no perceptible decline in peak absorbance by retinol, and a decline in peak UV absorbance by retinyl palmitate of only 1%. The results of the UV transmittance analyzer studies are summarized graphically in Figure 7. The normalized spectra for the retinol and retinyl palmitate formulations, before and after UV irradiation, are found in Figures 8 and 9.
Discussion
The chromophoric nature of retinoids not only gives them their color, it also subjects them to photodegradation via a number of previously referenced pathways, all of which destroy the integrity of the retinoids and almost certainly their ability to function biologically as intended. There may be other consequences as well. For example, some photochemical studies have found that excitation of retinol by UVR may generate highly reactive species that have been shown to damage cellular targets, including DNA and lipids.24, 25 This photo-induced degradation is made graphically clear in Figure 8 and Figure 9, which show the UV absorbances of compositions containing 0.1% retinol and 0.25% retinyl palmitate before and after exposure to UV radiation. Other studies have suggested a connection between topically applied retinoids, including the retinoid esters such as retinyl palmitate, and phototoxicity and photosensitivity.26, 27
While there are measurable benefits to including topically applied retinoids in a medical- or self-prescribed skin care regimen, the long-term effects of topically applied retinoids on humans are currently unknown. What is known is that photo-irradiation of retinoids induces chemical changes to them that, in addition to producing photodecomposition products, can generate reactive oxygen species, induce lipid peroxidation, and possibly cause DNA damage.28 Potential or possible toxicity aside, it seems evident that preserving retinoids from photodegradation is desirable, if for no other reason than to ensure their efficacy after application by the patient or consumer.
Numerous investigators have studied the photochemistry of retinoids extensively during the past 60 years. The details are specific to the retinoid and its immediate environment. In general terms, as with all chromophores, photon absorption by a retinoid leads initially to a singlet-excited state. This may be followed by a rapid return to the ground state directly from the singlet-excited state via internal conversion or fluorescence, or possibly by intersystem crossing to a triplet-excited state. It is during this triplet state that the retinoid assumes a diradical character, increasing the likelihood that a destructive (to the retinoid) chemical reaction may occur. If oxygen is present, superoxide anions may be formed; in the absence of oxygen, formation of radical anions and photoaddition reactions are possible.12
The observation made during these studies that retinol fluoresces—i.e., emits light under UV irradiation, is more than a photophysical curiosity. It points to the possibility that in the presence of the right partner compound, retinol may be induced to return to its more photochemically stable ground state before a destructive event can occur. In molecular and cell biology, compounds that fluoresce and compounds that stop or quench their fluorescence are known as fluorophore-quencher pairs. Such pairs are valuable tools for making sensitive measurements in biological samples. The process by which the quenching takes place is known as fluorescence (or Förster) resonance energy transfer. As shown in Figure 3, retinol fluorescence is strongly quenched by EHMC; in other words, retinol and ethylhexyl methoxycrylene make an excellent fluorophore-quencher pair.
In photochemical terms, one would say that retinol in its excited state becomes a donor that transfers its excited-state energy to the acceptor, EHMC, which in turn dissipates its new, excited-state energy nondestructively. This fortuitous finding is the reason that formulations containing these retinoids and EHMC exhibit remarkable photostability. These studies have employed and reported two methods to determine retinoid concentration before and after irradiation, and in the absence and presence of the photostabilizer EHMC. Whether measuring retinoid concentration directly by HPLC or indirectly in the UV transmittance analyzer studies, the results support the severe photodegradation of retinoids reported elsewhere by other investigators.
But while the two methods agree qualitatively, they differ quantitatively. The HPLC studies show that the nonphotostabilized retinoids experience significantly less photodegradation than the UV transmittance analyzer studies. Similarly, the UV transmittance analyzer studies show marginally greater photostability in the formulations with 4% EHMC than do the HPLC studies. By both methods, the formulations with 5% EHMC show the retinoids approaching near perfect photostability. Currently, no explanation has been found for the disparities in the results of the two methods.
One final observation before concluding. The first time one of the retinoid formulations was made without EHMC, HPLC analysis revealed significant loss of the retinoid before irradiation; this loss was on the order of 60%. This apparently happened despite the considerable care taken during preparation to protect the retinoid from exposure to light. The formulation was reproduced completely in the dark, which seemed to solve the problem. No such difficulties were encountered with any of the retinoid formulations containing 4% ethylhexyl methoxycrylene. Though anecdotal, this experience leads to the question of whether EHMC might be just as valuable for protecting retinoids during manufacturing as it is to preserve them after application to the skin.
Conclusions
Retinol and retinyl palmitate are clinically important retinoids that are widely used in topically applied overthe- counter skin care products. Both are highly photolabile or photo-unstable and exhibit rapid photodegradation, i.e. loss of concentration upon UV irradiation, resulting in an irreversible loss of efficacy. When combined in formulations with 5% EHMC, retinol and retinyl palmitate are photostabilized and show no loss of concentration, even after exposure to 5 MED.
Given the importance of delivering retinoids to patients and consumers in topical products, and the uncertainty of the long-term effects of the photodecomposition products of retinoids, there is no question that retinoids must be photostabilized and that EHMC presents an effective choice to achieve this outcome.
Acknowledgments: The authors gratefully acknowledge the following individuals for their invaluable assistance in the preparations of this article: Anna Pavlovic, Steve Semlow and Gary Wentworth of The HallStar Company; and Bob Drake of Drake Creative.
References
Send e-mail to [email protected].
1. T Yoshizawa, Molecular basis for color vision, Biophys Chem 50 17–24 (1994)
2. SS Tulachan et al, All-trans retinoic acid induces differentiation of ducts and endocrine cells by mesenchymal/epithelial interactions in embryonic pancreas, Diabetes 52 76–84 (2003)
3. F De Luca et al, Retinoic acid is a potent regulator of growth plate chondrogenesis, Endocrine 141 346–353 (2000)
4. TM Underhill, AV Sampaio and AD Weston, Retinoid signaling and skeletal development, Novartis Found Symp 232 171–188 (2001)
5. RD Semba, The role of vitamin A and related retinoids in immune function, Nutr Rev 56 S38–48 (1998)
6. B Lefebvre et al, Down regulation of the tumor suppressor gene retinoic acid receptor b2 through the phosphoinisotide 3-kinase/Akt signaling pathway, Molec Endocrin 20 2109–2121 (2006)
7. P Reilly and JJ DiGiovanna, Retinoid chemoprevention in high-risk skin cancer patients: Retinoids and cancer, Dermat Nurs 16 (2004)
8. IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN) website, Nomenclature of Retinoids (1981), www.chem.qmul.ac.uk/ iupac/misc/ret.html (accessed Sep 3, 2010)
9. A Valquist, S Kuenzli and J-H Saurat, Retinoids, in: K Wolff, L Goldsmith, SI Katz, BA Gilchrest, AS Paller and DJ Leffell, eds, Fitzpatrick’s dermatology in general medicine, 7th edn, McGraw-Hill, New York, ch 229 (2008)
10. WS Blaner and JA Olson, Retinol and retinoic acid metabolism, in: MB Sporn, AB Roberts and DS Goodman, eds, The retinoids: Biology, chemistry and medicine, 2nd edn, Raven, New York (1994) pp 529–542
11. T Perlmann, Retinoid metabolism: A balancing act, Nature Genetics 31 7–8 (2002)
12. WH Tolleson et al, Photodecomposition and Phototoxicity of Natural Retinoids, Int J Environ Res Public Health 2 147–155 (2005)
13. J Boehnlein, A Sakr, JL Lichtin and RL Bronaugh, Characterization of esterase and alcohol dehydrogenase activity in skin. Metabolism of retinyl palmitate to retinol (vitamin A) during percutaneous absorption, Pharm Res 11 1155–1159 (1994)
14. H Ji and B Seo, Retinyl palmitate at 5% in a cream: Its stability, efficacy and effect, Cosm & Toil 61–68 (114)
15. PP Fu et al, Photoreaction, phototoxicity and photocarcinogenicity of retinoids, Environ Carcinogen Ecotoxicol Rev 21 165–197 (2003)
16. AM Reddy and VJ Rao, Ionic photodissociation of polyenes via a highly polarized singlet excited state, J Org Chem 57 6727–6731 (1992)
17. G Crank and MS Pardijanto, Photo-oxidations and photosensitized oxidations of vitamin A and its palmitate ester, J Photochem Photobiol A 85 93–100 (1995)
18. R Teraoka, Y Konishi and Y Matsuda, Photochemical and oxidative degradation of the solid-state tretinoin tocoferil, Chem Pharm Bull 49 368–372 (2001)
19. LE Lamb, M Zareba, SN Plakoudas, T Sarna and JD Simon, Retinyl palmitate and the blue-light-induced phototoxicity of human ocular lipofuscin, Arch Biochem Biophys 393 316–320 (2001)
20. PP Fu, Q Xia, LG Blankenship, PJ Webb, WG Wamer and PC Howard, Photostability and photogenotoxicity of retinyl palmitate, 42nd SOT Annual Meeting abstract 1007 (2003)
21. S Isoe, SB Hyeon, S Katsumura and T Sakan, Photooxygenation of carotenoids. II. The absolute confi guration of loliolide and dihydroactinidiolide, Tetrahedron Lett 25 2517–2520 (1972)
22. Q Xia et al, Photo-irradiation of retinyl palmitate in ethanol with ultraviolet light—Formation of photodecomposition products, reactive oxygen species and lipid peroxides, Int J Environ Re. Public Health 3(2) 185–190 (2006)
23. C Bonda, A Pavlovic, K Hanson and C Bardeen, Singlet quenching proves faster is better for photostability, Cosm & Toil 125 40–48 (2010)
24. PP Fu et al, Photodecomposition of vitamin A and photobiological implications for the skin, Photochem Photobiol 83 409–24 (2007)
25. Q Xia et al, Formation of reactive oxygen species and lipid peroxides from photo-irradiation of retinyl palmitate and its photodecomposition products, The 32nd Annual Meeting of the American Society for Photobiology abstract 164 (2004)
26. J Ferguson and BE Johnson, Retinoid associated phototoxicity and photosensitivity, Pharmac Ther 40 123–135 (1989)
27. J Yan et al, Photoclastogenicity of retinyl palmitate and its photodecomposition products, The 32nd Annual Meeting of the American Society for Photobiology abstract 165 (2004)
28. WH Tolleson et al, Photodecomposition and phototoxicity of natural retinoids, Int J Environ Res Public Health 2 147–155 (2005)