Methods of active ingredient delivery for the personal care industry have undergone numerous improvements and advancements in recent years. Yet, one of the most enduring challenges has been how to deliver active components efficaciously in a wash-off personal care product that will not be removed by surfactants. The primary obstacle to achieving this has been in finding a method that will allow active ingredients to be attracted to the skin and, if necessary, penetrate slowly.
Throughout the years, advances in technology have enabled ten-fold improvements in delivery, which is evident in the polymer science currently being employed for body washes, face washes and other surfactant-based systems; however, these advancements have proven insufficient for delivery of active ingredients with the same ease and efficacy that can be realized through delivery by wash-off products. In today’s fast-paced society, where consumers continue to demand innovations that enhance convenience, there is a need for technology that can deliver a two-in-one benefit such as the effective delivery of active ingredients in a surfactant-based formulation.
Charged Vesicles
The described need can be met by taking advantage of the fact that active ingredients contained within a cationic molecular complex will attract to skin and hair and cannot be readily washed off. To accomplish this, a component must be found in that complex to facilitate attachment.
Fortunately, researchers have developed many types of vesicles that can withstand the surfactant environment and efficiently deliver the desired charge to achieve attachment. Charged molecules can offer electrostatic properties in proper environments, while polymers, as well as acids or even surfactants, can sometimes offer this convenience for certain delivery systems.
Silanes, or silica, have been shown to provide a highly effective manner for distributing this charge.1 Charging the silica in a capsule can improve the properties of skin adhesion. Additionally, other metals may offer the same ability and effectively deliver the active ingredients trapped within. By engineering the capsule, the structure of this highly charged vesicle can be made ready for delivery and can be given the capability to provide varied penetration, even in a surfactant environment (Figure 1). This, however, is dependent upon choosing the proper surfactants. Testing of formulations has revealed that lower pH ranges offer greater substantive capabilities (see pH and Substantivity). They also provide excellent stability in formulations with ammonium-based surfactants.
The benefits associated with these types of vesicle are quite obvious. They are very resistant to shear and temperature. In addition, they offer greater deposition properties due to their highly cationic charges. Furthermore, they have the capability to deliver both hydrophilic and hydrophobic ingredients, enabling a wide range of opportunities.
Wash-on Delivery System
A threefold delivery system appears to be the best approach for achieving delivery of active ingredients within a surfactant medium. A film former, a capsule and a polyquaternium, used in conjunction, offer greater substantivity than the use of any of these components individually.
In 2006, Aquea Scientific Corp. introduced a threefold technology to deposit cosmeceutical actives efficaciously onto skin and hair via surfactant products such as face wash, body wash, shampoo or conditioning products. The actives are washed on for delivery. The patented systema used to deliver them will be referred to as a wash-on system in this article to distinguish it from the rinse-off and leave-on approaches already in use.
The wash-on system encapsulates active ingredients into micron-sized particles. The particles then are given a positive charge, enabling them to be attracted to negatively charged skin and hair where they remain attracted throughout the day. These particles are not felt on the skin and will not affect application of moisturizers, cosmetics and styling products.
Delivering sunscreen actives: Initial patents2–4 describing this threefold technology addressed delivery of sunscreen actives. Studies have shown that varying amounts of organic and inorganic sunscreen can be delivered through this type of system, dependent upon the ratios of the three components used.
The ideal system would consist primarily of:
• a polyquaternium;
• a polyethelyne glycol (PEG);
• an encapsulated UV filter (e.g., ethylhexyl methoxycinnamate);
• an additional UVB filter (e.g., octocrylene);
• an additional UVA filter (e.g., avobenzone);
• siliconated titanium dioxide;
• film-formers, such as petrolatum; and
• possibly other types of semi-occlusive or porous film-formers.
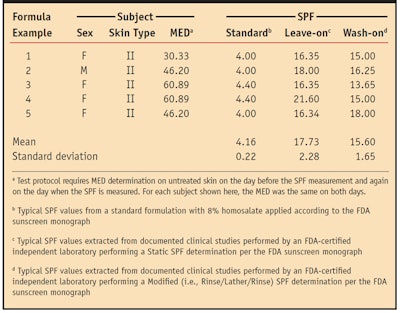
The deposition efficacy, as indicated by SPF, varies according to how these ingredients are combined, as demonstrated in Table 1. The table shows examples from several documented clinical studies using differently formulated products on subjects with similar skin types. In each study the formulation was applied at 13% in a leave-on product and in a wash-on product and compared to a standard formulation containing 8% homosalate. The correlation between leave-on deposition and wash-on deposition was defined by SPF testing. The procedure for this test consisted of wetting the skin with 10 mL of water dispensed via a syringe followed by application of the test formulation delivered to the site at a concentration of 2.0 mg/cm2. The lather was worked in for two minutes. After 30 sec, the site was rinsed with 20 mL of water and patted dry. The test material was allowed to dry for 15 min before exposure. Table 1 shows that the wash-on SPFs were only 12% lower than the leave-on SPFs, based on the means in this extracted data.
A typical leave-on sunscreen formulation containing approximately 13% w/w of the sunscreen actives achieves an SPF of 15 to 17. A wash-on formulation with an active concentration of 12–14% w/w added to a surfactant-based system at a 35% concentration leaves a residual SPF of anywhere from 12 to 15 after rinse, in an ammonium-based surfactant. This shows that the technology can deliver high amounts of the active ingredients with very little loss of the sunscreen.
Delivering non-sunscreen actives: Applications can go beyond sunscreen technology into hydrophilic and hydrophobic active delivery on demand in a wash-type environment.
A wide range of non-sunscreen actives may be delivered via the wash-on delivery system. Nonlimiting examples cited in a patent6 include bug repellents, acne treatments, bronzers, skin lighteners, antioxidants, deodorant compounds, hair growth inhibitors and stimulants, antimicrobials, corticosteroids, antiwrinkle actives and vitamins. The active can be provided as is or in a capsule. Within a single composition, multiple actives can be delivered, each of which may be encapsulated or nonencapsulated, in any combination.
In preferred embodiments, the active is encapsulated in sol-gel microcapsules, such as silica sol-gel microcapsules, which may also contain a cationic polymer. In other embodiments, the sol-gel microcapsules may be used with additional encapsulated active ingredients that enhance their barrier function. A sol-gel is a colloidal suspension that can be gelled to form a solid.
The microcapsules can be prepared so they experience no breakage or minimal breakage when applied to the skin. Alternatively, the microcapsules can be prepared so they experience various degrees of breakage, on average, when applied. Furthermore, they may be formulated so as to break open in response to conditions that occur on the skin or hair, so that after application the microcapsules act to release their contents in a time-release or controlled manner. Nonlimiting examples of these triggering conditions include pH, temperature, friction, pressure, and exposure to light or air.
Capsules used for delivering the active subtopically can be engineered to open on demand or to stay deposited on top of the skin through dissolving films, salt interaction, pH variability, or even a lipase mechanism. The capsule’s size determines the delivery depth. The capsule’s porosity controls the rate of deposition. The threefold technology has been shown to act differently on various capsules, depending on the net charge (i.e., the cationic environment in which the capsule is dispersed).
The key to proper delivery and attachment is in the creation of a highly charged environment with an electrostatic charge via polyquaternium or other positively charged component, creating a system with a pH of approximately 4.5 to 6.0. The interior of the capsule is engineered to withstand hydrophilic and hydrophobic actives and to open on demand.
Conclusion
With the new technology described here, the cosmetics and personal care industry now has the means by which to deliver active ingredients, heretofore limited to leave-on systems, in a surfactant-based environment. The ability to deliver highly effective products through a convenient and well-known category just got easier.
Acknowledgments: Aquea Scientific was honored as the 2006 recipient of Cosmetics & Toiletries magazine’s International Technology Award for Best New Technology. To nominate a technology or marketing launch for the 2007 awards, or for more information, send e-mail to [email protected].
References
1. DG Grier, Effective charge of glass and silica in deionized solutions (Jul 25, 2001) (www.physics.nyu.edu/grierlab/charge5b/node2.html, Accessed Jan 18, 2007)
2. US Pat 6,998,113, Bodywashes containing additives, DH Traynor, SM Markowitz, DL Compton, HG Traynor and M Dulak, assigned to Aquea Scientific Corp, USA (Feb 14, 2006)
3. US Pat 7,001,592, Sunscreen compositions and methods of use, DH Traynor, SM Markowitz, DL Compton, HG Traynor and M Dulak, assigned to Aquea Scientific Corp, USA (Feb 21, 2006)
4. US Pat 7,025,952, Methods of preparation and use of bodywashes containing additives, DH Traynor, HG Traynor, SM Markowitz and DL Compton, assigned to Aquea Scientific Corp, USA (Apr 11, 2006)
5. 21CFR Part 352, Subpart D, Federal Register 64(98) (May 21, 1999) Proposed Amendment: Docket 78-N0038/CP12 (Jun 21, 1999)
6. US Pat 7,037,513, Bodywash additives, DH Traynor, HG Traynor, SM Markowitz and DL Compton, assigned to Aquea Scientific Corp, USA (May 2, 2006)