A hexapeptide was developed based on the human skin type I collagen sequence. Its activities on skin biomarkers, procollagen I and hyaluronic acid release, and for dermal matrix protection and facial contouring benefits, were tested as described here.
Human type I collagen plays an essential function in skin health and regeneration. It is the most abundant protein, comprising 90% of the human body's protein content, and gives structural support to tissues like skin, tendons and bones.1, 2 In the skin, it constitutes a significant portion of the extracellular matrix (ECM), granting skin integrity and function and contributing to its biomechanical properties.2
Type I collagen is crucial for maintaining skin elasticity, firmness and an overall youthful appearance by promoting fibroblast cell proliferation, collagen synthesis and elastin production.3 Additionally, its synthesis is vital for skin health, as its decrease with aging contributes to manifestations like wrinkles and reduced elasticity. Scientific studies support the notion that collagen decline plays a significant role in skin aging, acting as both a cause and an effect of the process.4
Collagen-based supplements have been shown to repair skin damage and rejuvenate aging skin.5 Research indicates that peptides derived from human type I collagen can enhance wound healing, improve skin conditions and reduce wrinkles, making them highly valuable in cosmetics and regenerative medicine.6
The physicochemical properties of human type I collagen differ from those of animal-derived sources, affecting tissue outcomes and cellular responses. This highlights the importance of using peptides analogous to human collagen for specific skin regeneration applications.7 Today, the use of non-animal derived type I collagen is increasingly relevant in the cosmetic market due to the demand for sustainable sourcing8 and ethical concerns regarding animal testing.9 As a result, alternative sources such as recombinant collagen, collagen from marine byproducts and recombinant human type I collagen expressed in tobacco plants are gaining popularity,10 although these can raise safety concerns.
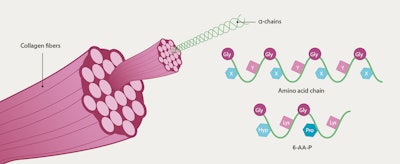
Non-animal derived peptides based on human proteins offer a promising alternative for cosmetic formulations, providing a safe and effective option for skin care and beauty products. Consequently, a non-animal derived hexapeptide (6-AA-P) was developeda, featuring the characteristic α-chain triplet sequence Gly-X-Y of the native human skin type I collagen, along with its most abundant amino acids: Gly-HyP-Lys-Gly-Pro-Lys (see Figure 1 below).
This design allows for easier skin penetration, compared with larger molecules, while the presence of Lys enhances solubility. Encapsulated in liposomes for stability and delivery into deeper skin layers, and spray granulated on maltodextrin, the biocompatible peptide replicates damaged collagen fragments to act as a triggering molecule. This potentially activates the skin’s regenerative processes, encouraging fibroblasts to produce more collagen and ECM components such as hyaluronic acid (HA).
The present work describes in vitro tests to determine the ingredient’s effects on biomarkers relevant to skin physiology, its impact on procollagen I and hyaluronic acid release, and its capability to protect against dermal matrix degradation. In addition, the ingredient was tested in vivo for facial contouring benefits in a cream applied by 23 volunteers twice daily for 42 days.
Material and Methods
Gene expression analysis: Normal human dermal fibroblasts were seeded in 24-well plates and cultured for 24 hr in culture medium. The medium was then replaced by assay medium containing or not (control) 0.1% 6-AA-P or the reference TGF-β, tested at 10 ng/mL, and the cells were incubated for 24 hr. The total extracted RNAb of each condition was reverse transcribed (RT-qPCR performed). The expressionc of give genes important in skin physiology was then determined and their expression was normalized to the housekeeping genes RPS28, GAPDH and B2M.
Procollagen I and hyaluronic acid release in fibroblasts: Human dermal fibroblasts were obtained from a donor and cultured in a monolayer until confluency. The cells were then exposed to a 1% solution of the collagen peptide for 48 hr and compared with untreated cells (control). As a further control, cells were treated with 3 µg/mL hydroxyproline, a major component of the collagen protein. After treatment, a sample of the cell culture supernatant was harvested and the amount of procollagen I released by the cells was quantified by ELISA. For HA release, the cells were exposed to 0.1% or 0.3% collagen peptide solution or not (control), or the positive reference compound TGF-β (10 ng/mL) for 72 hr. HA released in the culture supernatants was measured by ELISA.
Dermal matrix support: Human explants from a 28-yr-old donor were treated with a test cream containing 2% 6-AA-P or the placebo cream (control) for a total of four days. To evaluate the ingredient’s "Novel Coll effects on skin aging, the explants were pre-treated with the test products for 24 hr before enzymatic digestion using collagenased according to a novel ex vivo skin model (proprietary) from a third partye.
In short, skin explants were incubated with a solution of collagenase diluted in HBSSf to obtain only partial digestion of the dermal collagens. After the digestion, the explants were post-treated once daily with the products for three days. After treatment, the explants were fixed in formalin, embedded in paraffin and sectioned for collagen labelling. To evaluate the dermal structure, a collagen-specific staining with Sirius Redd was performed and analyzed under transmitted lightg. The histological staining images were analyzed and the average intensities (corresponding to collagen levels in the dermis) were quantified.
In vivo efficacy: Twenty-three female volunteers (ages 50 to 62) with signs of skin aging applied a cream containing 2% 6-AA-P or the corresponding placebo to each side of the face twice daily. At the beginning of the study (day 0) and after 42 days, the smoothnessh, elasticityj, collagen fiber density and perimeterk, and thicknessm of the skin were assessed. The facial contourn and the skin dynamic firmness (analyzed with a high-speed camerap) were also evaluated after the application period.
In vitro, Ex vivo Results and Discussion
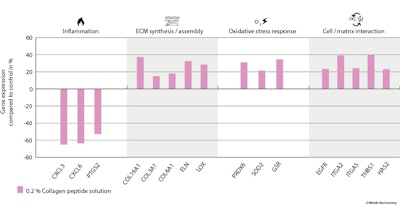
Gene expression analysis: Investigating the effect of 6-AA-P on the expression of genes important in skin physiology revealed the ingredient induced the upregulation of genes involved in the ECM synthesis and/or assembly, such us COL3A1, COL16A1, COL6A1, VCAN, ELN, HAS2, LOX, LOXL2 and SDC4. Genes involved in cell-matrix interaction and related to growth factors (EGFR, ITGA2, ITGA5, THBS1 and TGFBI) were also upregulated by 6-AA-P.
In contrast, 6-AA-P downregulated the genes CXCL1, CXCL3, CXCL6 and PTGS2, which are all related to inflammation, and it upregulated genes involved in oxidative and cellular stress response, including PRDX3, PRDX6, SOD2, GSR, CAT and MT1G (see Figure 2 below). The housekeeping gene GAPDH, which was used to normalize the results, was not affected by 6-AA-P.
Taken together, the gene expression results showed that 6-AA-P induces cellular responses like the repair process, cell adhesion, and extracellular matrix synthesis and assembly while inhibiting inflammation. The upregulation of genes expressing antioxidant enzymes enhances the skin’s defense against oxidative stress, protecting skin cells from damage caused by free radicals and environmental factors.
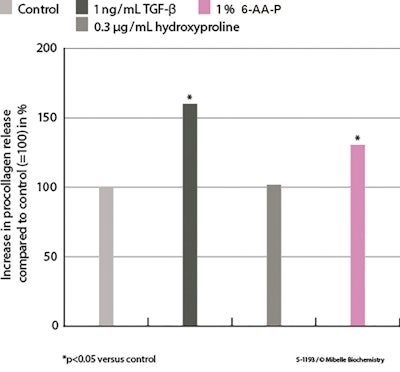
Effects of 6-AA-P on procollagen I release: In dermal fibroblasts treated with 1% 6-AA-P, ELISA showed that treatment significantly increased pro-collagen I synthesis by 29.7%, compared with the control (see Figure 3 below). Intriguingly, treatment with hydroxyproline, a major fragment of collagen itself, had no effect. In summary, it is the specific peptide sequence described in this study that induced procollagen I synthesis and release, which is crucial for maintaining the skin’s structural integrity.
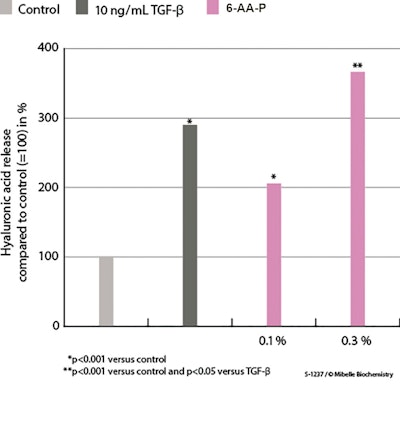
Effects of 6-AA-P on hyaluronic acid release: In dermal fibroblasts treated with 0.1% and 0.3% 6-AA-P, ELISA revealed the stimulation of HA release by 102% and 263%, respectively, compared with the control (see Figure 4 below). Consequently, the 0.3% collagen peptide solution outperformed the increase in HA release induced by treatment with the positive reference TGF-β. By maintaining moisture levels, HA contributes to skin’s elasticity and firmness, among other known benefits.
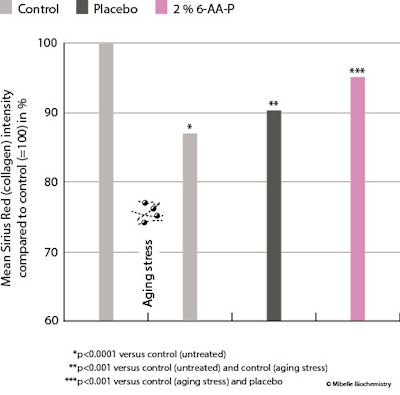
Dermal matrix support: Since collagen levels decrease during aging, which leads to skin sagging and the appearance of wrinkles, preserving collagen levels and the collagen network is a valid anti-aging approach. Thus, as stated, to investigate the ingredient’s effects on these factors, skin explants were pre-treated with either a placebo cream or a cream containing 2% 6-AA-P for four days, followed by collagenase degradation as a stress aging model.
The untreated control explants exhibited a strong red color, indicating an intact dermal structure. However, stress aging caused noticeable matrix degradation, including visible breaks and poor-quality dermal collagen fibers (see Figure 5, left, below). Quantification of Sirius Red intensity showed that aging stress reduced dermal collagen to 87% in the aged control, compared with 100% in the control.
In contrast, treatment with 2% 6-AA-P after stress aging preserved 96% of dermal collagen (see Figure 5, right). The placebo treatment resulted in a collagen content reduction to 90%. Thus, the collagen loss from aging was significantly prevented by 2% 6-AA-P but not by the placebo, as shown by the ex vivo skin model simulating aging, demonstrating the ingredient’s ability to effectively protect dermal collagen fibers to maintain a well-organized dermal structure.
In vivo Results and Discussion
As stated, a randomized, placebo-controlled clinical study (n = 23) was carried out to investigate the efficacy of 6-AA-P on various skin parameters that indicate signs of facial aging. Subjects applied a cream containing the test ingredient or a corresponding placebo to each side of the face twice daily for 42 days.
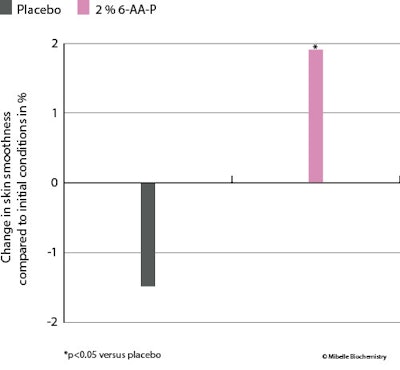
Skin smoothness: Skin smoothness was assessed by a stereo fringe projection systemh that robustly characterizes the skin micro-relief and topography by quantifying the average profile heights of the skin, thereby indicating the overall unevenness of the skin surface. Treatment with 2% 6-AA-P significantly increased skin smoothness by 2% (vs. baseline) whereas with the placebo, smoothness decreased by 1.5% (see Figure 6 below). The 6-AA-P ingredient therefore provided a more uniform skin texture.
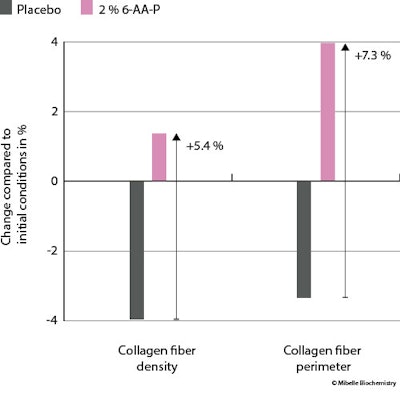
Collagen fiber density and perimeter: To assess the skin collagen fiber condition, confocal microscopy was used to investigate collagen fiber density and perimeterk. Treatment with 2% 6-AA-P increased the collagen fiber density by 1.4% vs baseline – amounting to a density increase of 5.4% over the placebo, where density decreased by 4% (see Figure 7 below). In addition, the use of 6-AA-P enhanced the collagen fiber perimeter by 4%, registering 7.3% increase over the placebo, where fiber perimeter decreased 3.3%.
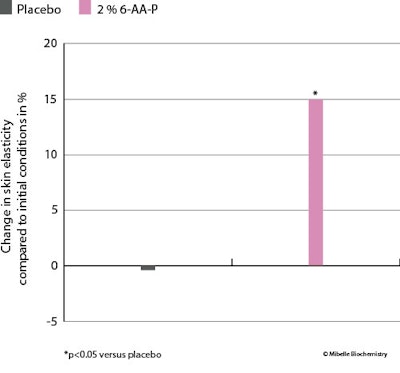
Skin elasticity: Furthermore, skin elasticity was evaluated with a cutometerj, which found that the application of 6-AA-P increased skin elasticity by 15% whereas the placebo showed no affect. This improvement in elasticity was significant compared to the placebo (see Figure 8 below).
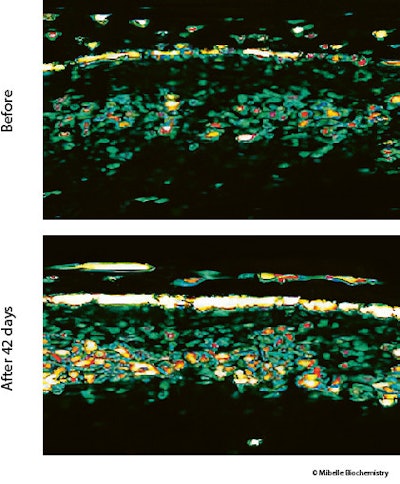
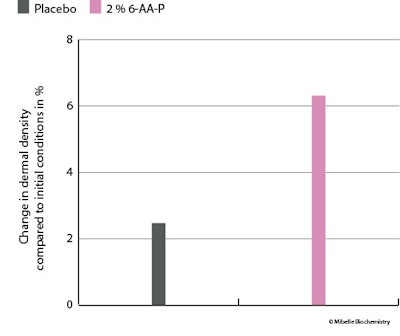
Dermal thickness and density: Skin dermal thickness and density were assessed using ultrasonography analysis. An improvement is visibly demonstrated with the application of 6-AA-P, which promoted denser regions within the dermis (see Figure 9 below); quantification of the ultrasonography signal revealed a 6.3% increase in dermal density (see Figure 10 below).
Facial contours and dynamic firmness: A lax dermis often appears as saggy and lacking the firmness and elasticity of youthful skin. So to determine the effects of 6-AA-P on such facial contours, the dynamic firmness of facial skin was assessed, focusing on how quickly skin returned to its original position after subjects made facial expressions.
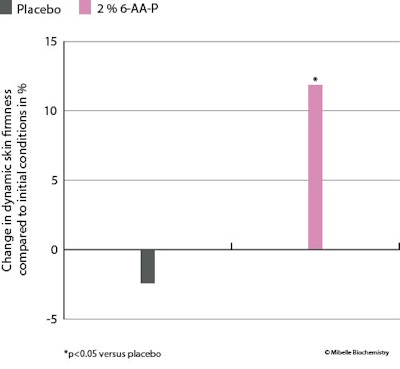
Using a high-speed camera, the retraction time of skin on the lower face was measured after a standardized expression was made, both at the start and after six weeks of product application. At 2% in the test formula, 6-AA-P improved facial dynamic firmness by 12% after the six weeks’ application, while the placebo treatment resulted in a 2.4% decrease in firmness (see Figure 11 below). The improved skin bounce-back time demonstrates 6-AA-P's effectiveness in boosting facial skin's elasticity, to in turn improve tensile strength and resilience, crucial for maintaining a toned and youthful appearance.
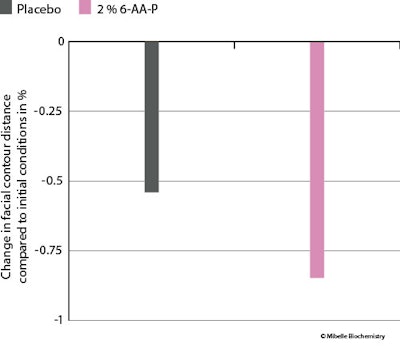
Lastly, the facial contour, measured by the mean contour distance, was evaluated after obtaining photographsn. Treatment with 6-AA-P reduced the facial contour by 0.8% (see Figure 12 below).
In summary, the clinical data highlighted 6-AA-Ps efficacy in stimulating collagen, thereby enhancing dermal density, firmness, elasticity and overall skin dynamics.
Conclusions
This study introduces a bio-activating human collagen type I peptide (6-AA-P)a as a novel ingredient to impart youthful skin properties. It has a sequence that corresponds to the characteristic human skin collagen type I and addresses the ethical and safety issues associated with animal-marine-recombinant-derived materials.
Overall, the in vitro study results presented here show that the sequence in 6-AA-P stimulates procollagen and HA release, and it acts as a trigger for skin repair mechanisms. This is in line with current research indicating that collagen decline plays a significant role in the aging process, rather than solely being an effect of aging – which makes collagen boosting a promising longevity intervention.
The results of the in vivo study described here show that 6-AA-P enhanced the skin's regenerative and repair capabilities. It positively impacted signs of skin aging by stimulating collagen production, increasing dermal density, firmness and elasticity. Additionally, it improved facial skin dynamics and exhibited an anti-sagging effect, contributing to skin remodeling.
In summary, the biocompatible hexapeptide presented emerges as a novel solution for comprehensive skin remodeling and rejuvenation, positioning it as a key player in anti-aging treatments and paving the way for future skin care innovations.
Footnotes
a CollPerfect P6 (INCI: Collagen Amino Acids) is a product of Mibelle Biochemistry.
b TriPure Isolation Reagent, Merck, Germany
c LightCycler System, Roche Molecular System Inc., Switzerland
d Sigma-Aldrich, USA
e Syntivia, Toulouse, France
f Dutscher, France
g Zeiss Axiovert, Carl Zeiss, Germany
h Stereo-Fringe projection system, AEVA-HE, Eotech, United States
j Cutometer dual MPA 580, Courage+Khazaka Electronic GmbH, Germany
k Confocal Microscopy Vivascope 1500, Mavig, Germany
m Dermascan C system, Cortex technology, Denmark
n VECTRA-XT, Canfield, United States
p Sony Corp., Japan
References
- Baltazar, T., et al. (2022). Native human collagen type I provides a viable physiologically relevant alternative to xenogeneic sources for tissue engineering applications: A comparative in vitro and in vivo study. J Biomed Mater Res B Appl Biomater, 110(10) p. 2323-2337.
- Hwang, S.J., et al. (2020). Human collagen alpha-2 type I stimulates collagen synthesis, wound healing and elastin production in normal human dermal fibroblasts (HDFs). BMB Rep, 53(10) p. 539-544.
- San Antonio, J.D., et al. (2020). Collagen structure-function mapping informs applications for regenerative medicine. Bioengineering (Basel), 8(1).
- Ewald, C.Y., et al. (2015). Dauer-independent insulin/IGF-1-signalling implicates collagen remodeling in longevity. Nature, 519(7541) p. 97-101.
- Reilly, D.M. and Lozano, J. (2021). Skin collagen through the life stages: Importance for skin health and beauty. Plastic and Aesthetic Research, 8.
- Naomi, R., Ridzuan, P.M. and Bahari, H. (2021). Current insights into collagen type I. Polymers (Basel), 13(16).
- Henriksen, K. and Karsdal, M.A. (2016). Type I Collagen. In Karsdal, M.A., ed., Biochemistry of Collagens, Laminins and Elastin, Academic Press, p. 1-11.
- Maistrenko, L., et al. (2022). Collagen obtained from leather production waste provides suitable gels for biomedical applications. Polymers, 14(21).
- Guerle-Cavero, R. and Balfagon-Costa, A. (2023). Study of elastin, hydrolyzed collagen and collagen-like products in a tri-layered chitosan membrane to test anti-aging skin properties. Int J Mol Sci, 24(13).
- Seror, J., et al. (2021). The potential use of novel plant-derived recombinant human collagen in aesthetic medicine. Plast Reconstr Surg, 148(6S) p. 32S-38S.