Consumers are educated about the damaging effects of excessive UV radiation on the health and appearance of skin. This has driven the medical community and consumers to demand increasing levels of protection from sun care products, in addition to improved emollient and anti-inflammatory properties.1–4
UVA and UVB radiation causes the direct absorption of photons by DNA and subsequent structural changes, in addition to the generation of reactive oxygen species (ROS), resulting in lipid peroxidation. Moreover, UV radiation impairs the human immune response by increasing the activity of suppressor T-cells.5 The effects of UVA and UVB radiation have created the need for sunscreen products. For example, several studies show that both UVA and UVB increase the production of matrix metalloproteases (MMPs) four- to five-fold, resulting in the remodelling and degradation of the extracellular matrix (ECM).6–9
The visible signs of aging or photo-aging and skin cancer resulting from UV radiation qualify it as the most dangerous aggressor among the permanent environmental insults on human skin.10-12 Therefore, it is necessary to develop photostable skin care products with broad spectrum protection and enriched with antioxidant and immunomodulant compounds.
The aim of the present work was thus to control the photoprotective activity of different sunscreen emulsions using a specialized carrier based on chitin nanofibrils (CN).13 CN are able to link active compounds via hydrogen bonds, thereby increasing the penetration of the compounds into the skin layers and increasing their specific activity.14 In the present study, CN were used as a carrier to enhance the photoprotection of sunscreens as well as ameliorate their antioxidant and immunomodulating activity.
Subject Criteria
In accordance with previous studies,15-17 40 female volunteer subjects aged 20–35 of skin types I–III and individual typology angle (ITA) values > 28 were randomly selected and enrolled in the study, using a colorimetera to avoid any possible bias toward higher SPF.18 Each volunteer was subjected to the same procedure controlled by an expert dermatologist for determination of the minimal erythema dose on unprotected (MEDu) and protected (MEDp) skin.
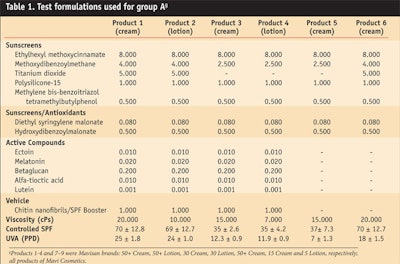
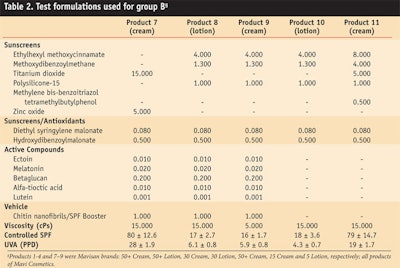
Written informed consent was obtained prior to testing and subjects who used tanning beds, took medication, were treating dermatological conditions, had a history of abnormal response to sun exposure, or were pregnant or lactating were excluded. Subjects were subdivided into groups A and B, with 20 women in each group. Both groups were treated with the test products and formulations shown in Table 1 and Table 2, respectively. Note that for comparison purposes, some of the formulations included CN as a carrier while others did not.
Determining SPF
In accordance with the treatment plan, 11 different sunscreen preparations (SSP) including eight creams and three lotions—following four sets of international fomulation standards—were distributed randomly over a test area on the middle back of each subject at the distance of 1 cm using a micropipette. The formulations were applied to a 6 x 25 cm2 area for group A and a 5 x 25 cm2 area for group B. The application area was smaller in group B due to limited space on subjects’ backs. In both groups, two standard cream formulations served as controls.
Using a weighed by loss technique, each product was applied to the assigned area at a rate of 2 mg/cm2 and gently spread in a series of small doses into the skin with a finger cot using medium pressure for 25 sec. The product was then left to dry for 20 min before subjects were exposed to UV radiation.
According to previous studies and COLIPA recommendations,15, 19, 20 a xenon arc lamp was used and filtered with colored glassb. The output of solar simulation was monitored daily with a 3D UV radiometerc. The two groups of subjects were exposed to doses of simulated UV light increased 1.25X under the solar simulator in the prone position at the controlled RH of 50% and temperature of 22°C.
The MEDu and MEDp were determined after 20 hr both by visual and colorimetric assessmenta, and the SPF was calculated according to the international COLIPA SPF Test Method.19,20 The results are shown in Table 1 and Table 2.
UVA Test Method
A delayed study was conducted one month later on groups A and B to determine the UVA protection factor of the same formulations. The test sunscreens were applied on the subjects’ backs at a concentration of 2 mg/cm2 as described previously but utilizing a different fluorescent lampd that was found by Mark et al.21
to provide a better broad spectrum UVA source. The new lamp was filtered by a 2-mm color glass filtere as a UVB blocker and placed in alternate positions in a sun tanning unit.14, 15
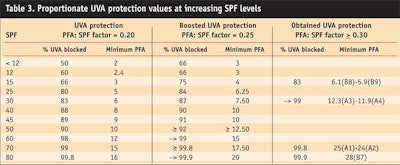
Exposure began 20 min after sunscreen application and the total exposure time was 50 min. For a series of timed and progressive doses, each one 25% longer than the previous, erythemal response was scored visually and by colorimetera ± 1 hr later. The obtained results were confirmed by use of the COLIPA PPD method. The data obtained is reported in Table 3, including the protection factor of UVA (PFA).
Statistical Evaluations
Statistical evaluations were performed with comprehensive, scientific graphing softwaref. All evaluations were conducted as two-tailed analyses with a minimum 95% confidence interval (p > 0.05) using an analysis of variance for repeated measures and a Tukey post-hoc test to determine statistically significant differences in the results.
Photostability
The absorption capacity of the sunscreen molecules and vehicles used and the related photostability of the final emulsions were verified according to the Bonda and Shaath method.22, 23 The triplet-triplet quenchers diethyl syringylene malonate (DSM) and hydroxydibenzoylmalonate (HDM) were initially included in the test formulas as photostabilizer compounds and thus were not included in the absorption and photostability calculations. Therefore, the polarity of the different vehicles was controlled, verifying the polarity and photo-decay of the final formulations.24
Protection Parameters
According to Urbach,25 the percentage ratio of damage from the UVB and UVA components in sunlight over the course of one day is 80:20. Of the 20% UVA damage (320–400 nm), 62% of the damage risk has been attributed to the shorter UVA II wavelengths (320–340 nm). Therefore, to provide proportional UVA and UVB protection, a sunscreen must protect against the 80:20 ratio of UVB/UVA incident sunlight.
Moreover, recent research reports the importance of protecting the skin against blue light (380–500 nm). While blue light is not considered detrimental to the skin, these wavelengths have been found to form 500X more free radicals than longer, red light wavelengths.26-28 Finally, other studies have shown that long-wave UVA (> 340nm) may contribute in different ways to premature skin aging, and that repeated exposure to suberythemal doses of UVA may result in long-term damage such as an increase in skin photoaging.29, 30
Results and Comments
Considering these factors, the test sunscreens shown in Table 1 and Table 2 all provided effective and appropriate proportional protection against UVA and UVB rays. Tables 1 and 2 also report the SPF values for UVB and UVA radiation, and it can be deduced that these sunscreens provided proportional broad spectrum UVB/UVA protection in accordance with views expressed by dermatologists worldwide.31
In Table 3, however, a comparison of the basic and proportioned UVA/UVB protection provided by the test sunscreens reveals that those formulated with CN as a carrier exhibited higher levels of UVA protection than the standard sunscreen formulations (emulsions 5, 6, 10 and 11). Whereas the standard formulas achieved a PFA of 0.19–0.25, the sunscreens formulated with CN measured a boosted PFA ≥ 0.34. Since the sunscreens with CN provided a higher PFA, they could be particularly useful to protect sensitive skin, or for individuals receiving dermatological treatments. In addition, in accordance with Japanese rules, these sunscreens may be labelled as PA+++.
This higher PFA in CN formulations is likely due to the right combination of the UVB/UVA sunscreens and the previously reported boosting activity of CN,32 which occurs upon binding to the SC.14, 32, 33 Also, when bound to lutein, melatonin, lipoic acid and/or ectoin in sunscreens, CN reinforce the photoprotective effects by inducing the formation of a film on the skin that imparts a thicker, more uniform application of the product,27, 28 thus improving the gradual absorption of the active compounds in the sunscreens. In addition, CN have been found to exhibit antioxidant, hydrating and immuno-protective effects.32,33
While these polyglucosides could be considered booster molecules for improving SPF, the mechanism behind their ability to boost PFA is unknown—although the author hypothesizes it could be due to increased light reflectance.
In conclusion, this natural polyglucoside may be used to enhance the antiaging activity of sunscreens, thus addressing increasing global consumer concerns.32, 34
References
1. NA Shaath, Evolution of modern sunscreen chemicals, in Sunscreens: Development, Evaluation, and Regulatory Aspects 2nd ed, NJ Lowe, NA Shaath and MA Pathak, eds, New York: Marcel Dekker (1997) pp 3–34
2. ET Kaye, JA Levin, IH Blank, KA Arndt and RR Anderson, Efficacy of opaque photoprotective agents in the visible light range, Arch Dermatol 127 351–355 (1992)
3. R Roelandts, Sheeding light on sunscreens, Clin Exp Dermatol 23 147–157 (1998)
4. S Bruening, M Leonard, R Kawa, U Issberner, and A Tomlinson, Role of emollients and emulsifiers in sunscreen formulations, in Sunscreens: regulations and Commercial development, 3rd ed, NA Shaath, ed, New York: Marcel Dekker (2005) pp 449–460.
5. P Hersey, G Haran, E Hasic and A Eduards, Alteration of T-cell subset and induction of suppressor T-cell activity in normal subjects after exposure to sunlight, J Immunol 131 171–174 (1983)
6. G Hermann, M Wlasheck , TS Lange, K Prenzel, G Goerz and K Scharffetter-Kochanck, UVA irradiation stimulates the synthesis of various matrix-metalloproteinases (MMPs) in cultured human fibroblast, Exp Dermatol 2 92–97 (1993)
7. P Brenneisen, J Wenk, LO Klotz, M Wlaschek, K Brivia, T Krieg, H Sies and K Scharffetter-Kochanck, Central role of ferrous-ferric iron in the ultraviolet B irradiation-mediated signalling pathway leading to increased interstitial collagenase (matrix-degrading metalloprotease (MMP)-1) and stromelysin-1 (MMP-3) mRNA levels in cultured human dermal fibroblasts, J Biol Chem 273 5279–5287 (1998)
8. P Brenneisen, J Oh, M Wlaschek, J Wenk, K Briviba, C Hommel, G Hermann, H Sies and K Scharffetter-Kochanck, Ultraviolet B wavelength dependence for the regulation of two major matrix-metalloproteinases and their inhibitor TIMP-1 in human dermal fibroblasts, Photochem Photobiol 64 877–885 (1996)
9. P Brenneisen, H Sies and K Scharffetter-Kochanck, Ultraviolet-B irradiation and matrix metalloproteinases: from induction via signalling to initial events, Ann NY Acad Sci 973 31–43 (2002)
10. C Elmets, Sunscreens and photocarcinogenesis: An objective assessment, Photochem Photobiol 63 435–440 (1996)
11. American Cancer Society, Cancer Facts and Figures (2002/2003)
12. TS Housman, SR Feldman, PM Willford, AB Fleischer Jr., ND Goldman, JM Acostamadiedo and GJ Chen, Skin cancer is among the most costly of all cancers to treat for the medicare population, J Am Acad Dermatol 48(3) 425–429 (2003)
13. P Morganti, RA Muzzarelli and C Muzzarelli, Multifunctional use of innovative chitin nanofibrils for skin care, J Appl Cosmetol 24 105–114 (2006)
14. P Morganti, G Fabrizi, P Palombo, M Palombo, E Ruocco, A Cardillo and G Morganti, Chitin-nanofibrils: A new active cosmetic carrier, J Appl Cosmetol (2008)
15. P Morganti, New data on skin photoprotection, Int J Cosmet Sci 22 305–312 (2000)
16. R Wolf, D Wolf, P Morganti and V Ruocco, Sunscreens, Clinics in Dermatol 19 452–459 (2001)
17. P Palombo, G Fabrizi, V Ruocco, E Ruocco, J Flühr, R Roberts and P Morganti, Beneficial long-term effects of combined oral/topical antioxidant treatment with carotenoids lutein and zeaxanthin on human skin: A double-blinded, placebo-controlled study in humans, Skin Pharmacol Physiol, 20 199–210 (2007)
18. P Elsner, Chromametry, Hardware, Measuring Principles and Standardization, in Bioengineering of the skin: cutaneous blood flow and erythema, E Berardesca, P Elsner and HI Maibach, eds, Boca Raton, FL: CRC Press (1995) pp 247–252
19. COLIPA (1994) Sun Protection Factor Test Method. COLIPA Publication 94/289:October 1994.
20. COLIPA (2002) COLIPA recommendation No. 11-SPF Classification/upper limit. COLIPA Document Reference 02/068-AF (June 2002).
21. R Mark and KL Gabriel et al, Testing sunscreens for UVA protection, Cosm & Toil 105 63–66 (1990)
22. C Bonda and DC Steinberg, A new photostabilizer and full spectrum sunscreens, Cosm & Toil 115(6) 37–45 (2000)
23. Ibid. Reference 1, 325
24. P Morganti, Unpublished data of MAVI laboratory (2008)
25. F Urbach, Ultraviolet A transmission by modern sunscreens: Is there a real risk? Photodermatol Photoimmunol Photomed 9 237–241 (1993)
26. P Morganti, G Fabrizi and C Bruno, Protective effects of oral antioxidants on skin and eye function SkinMED: Dermatology for the Clinician 3(6) 310–316 (2004)
27. P Morganti and G Morganti, Fotoprotezione e Luce Blu, Cosmetic News 30 225–228 (2007)
28. P Morganti, D Sousa Martins, and G Morganti, Skin Activity of Lutein, SÖFW-Journal 134(1–2) 2–11 (2008)
29. NJ Lowe, DP Meyers, JM Wieder, D Luftman, T Bourget, MD Lehman, AW Johnson and IA Scott, Low doses of repetitive UVA induce morphological changes in human skin, J Invest Dermatol 105 739–743 (1995)
30. RM Lavker, GF Gerberick, D Veres, CJ Irwin and KH Kaidbey, Cumulative effects from repeated exposures to suberythemal doses of UVB and UVA in human skin, J Am Acad Dermatol 32 53–62 (1995)
31. H Honigsmann, UVA and human skin, J Photochem Photobiol B 4 229–232 (1989)
32. P Morganti, G Morganti, G Fabrizi and A Cardillo, A new sun to rejuvenate the skin, J Appl Cosmetol 26 159–168 (2008)
33. P Morganti and G Morganti, Carotenoids and vitamins to prevent photodamage and skin aging, Eurocosmetics 15(3) 10–17 (2007)
34. G Biagini, A Zizzi, F Giantomassi, F Orlando, G Lucarini, M Mattioli Belmonte, MG Tucci and P Morganti, Cutaneous absorption of nanostructured chitin associated with natural synergistic molecules (Lutein) J App Cosmet 26 69–80 (2008)