Acne vulgaris is a common, chronic and recurring disease that involves multiple etiological factors including follicular hyperkeratinization, increased sebum production, Propionibacterium acnes proliferation and inflammation. It affects about 80% of teenagers and young adults and is most prevalent in those aged 12 to 24 years, although it can occur at all ages. As many as 17 million people are thought to be affected in the United States alone and the acne therapeutics market is forecast to show moderate growth in revenues through 2016; market data suggests the global acne market was worth US $2.8 billion in 2009 and is estimated to reach revenues of $3.02 billion by 2016. This moderate increase in revenue is attributed to an overcrowding of the market with generics and increased consumer acceptance of alternative therapies, although the acne therapeutics market is witnessing a shift toward combination products using two or more effective acne treatments.
One of the major causes of acne is the increase in sex hormones, especially androgens such as testosterone, which occurs during puberty. Testerone is converted in the skin to dihydrotestosterone (DHT) by α-reductase, which stimulates the sebaceous glands to enlarge and produce more sebum. The more sebum produced, the worse the acne will become. Further, a study by Lee et al. suggests that DHT may also be involved in the production of proinflammatory cytokines in acne.
Abnormal follicular keratinization is also involved in the development of acne vulgaris. The presence of unsaturated fatty acids in sebum alter the calcium dynamics in epidermal keratinocytes and induce abnormal follicular keratinization,4 and if sebum and keratin block the skin pores, they can cause comedones—small, skin-colored bumps or papules—to develop and hair follicle walls to rupture. Further, bacterial and comedonal debris cause acne pimples or pustules, i.e., inflammatory lesions.
Acne typically appears on the oil-producing areas of the body, namely the face, chest and back, and can have short-term, potentially lasting psychological effects such as decreased self-esteem and self-confidence leading to social withdrawal and even depression. While the three levels of acne severity are generally considered mild, moderate or severe, even mild acne can be troublesome, especially to teenagers who see each pimple as a major cosmetic challenge.
Interestingly, using a genetic-based strategy, Bek-Thomsen et al. recently demonstrated that follicles from healthy skin were exclusively colonized by P. acnes, whereas the follicular microbiota of acne patients included, in addition to P. acnes, Staphylococcus epidermidis and minor proportions of other species.5 However, previous studies excluded Staphylococci as agents that play a role in the pathogenesis of acne due to their rapid development of resistance to therapeutic antibiotics.6 The presence of S. epidermidis exclusively in acne-affected follicles raises the question of the potential role of this species in acne.
Treating Acne
There are four major targets presently governing acne therapy, including correcting the altered pattern of follicular keratinization; decreasing sebaceous gland activity; decreasing the follicular bacterial population, especially P. acnes; and producing an anti-inflammatory effect by inhibiting the production of extracellular inflammatory products through the inhibition of these microorganisms.7 These mechanisms are described in more detail in the Discussion and Results section later in this article.
Presently, oral or topical isotretinoin (retinoic acid) is the only single agent that is effective against all four major pathophysiologic features. However, when used at therapeutic doses, this drug is responsible for several serious side effects including teratogenicity8 due to interference of the exogenous retinoic acid with endogenous retinoic acid signaling, which plays a role in the patterning of developing embryos.9 Further, the use of broad-spectrum antibiotics such as erythromycin, clindamycin, etc., has led to widespread resistance. Therefore, retinoic acid should be used only in the most severe cases and alternative treatment approaches should be considered first.
The most common alternate approaches include exfoliating agents such as salicylic and glycolic acid but skin becomes more sensitive to sun-induced damage when exfoliating agents are used for long periods of time. Another common agent found in anti-acne products in the United States is benzoyl peroxide. It was previously listed as a Category III ingredient, meaning its safety was uncertain, but in 2010 it was reclassified as a Category I ingredient, meaning it is generally recognized as safe and effective. The recommendation now is that acne treatments be combined to target as many pathogenic factors as possible.10, 11
Bakuchiol and Acne
Meroterpene, especially bakuchiol (see Figure 1), has been reported to exhibit strong antibacterial effects.12, 13 In relation, a comparative DNA microarray study using bakuchiol and retinol, and bakuchiol’s anti-aging potential were recently reported by Chaudhuri.14, 15 Interestingly, the authors reported that Tazarotene-inducible gene 1 (TIG1) is significantly up-regulated by both bakuchiol and retinol, and the expression of TIG1 is found to be down-regulated in a variety of human cancers as well as acne, rosacea and psoriasis. Thus, it is quite conceivable to assume that the up-regulation of TIG1 gene by bakuchiol may provide a solution to problem skin.
This led the authors to further examine that material’s anti-acne activity in the described study, where a specific natural 95% pure meroterpene obtained from the edible seeds of Psoralea corylifoliaa, 4-[1E, 3S)-3-ethenyl-3, 7-dimethyl-1, 6-octadienyl (INCI: Bakuchiol) was evaluated to determine if it could effectively treat acne-affected skin.16, 17 In addition, the authors sought to determine if bakuchiol could be used in combination with salicylic acid. Assessments were made by evaluating the material’s effects on 5-α-reductase expression, antibacterial and antifungal inhibition, collagenase and elastase inhibition, and inflammation. Its clinical efficacy also was evaluated.
Materials and Methods
5-α-reductase expression: HaCaT human kerationocytes were used to study the effects of bakuchiol and retinoic acid on 5-α-reductase expression. Both materials were diluted in test concentrations ranging from 5 to 50 µg/mL in the culture media. At confluence, cells were trypsinized and seeded in microplates at a density of 50,000 cells per well. After a 4-hr incubation, the supernatants were discarded and new culture medium was added containing the test and control products, i.e., without bakuchiol or retinoic acid. After 48 hr of incubation at 37°C in an atmosphere containing 5% CO2, the supernatants were discarded and the cells were washed using sterile PBS. The expression of 5-α-reductase was then measured via an immunofluorescence technique using a specific antibody and evaluated spectrofluorometricallyb at excitation wavelengths of 485 nm and emission wavelengths of 530 nm.
Antibacterial and antifungal inhibition: To assess the antibacterial and antifungal activity of bakuchiol and salicylic acid, their inhibitory concentrations (IC50) against P. acnes first were defined as the points at which turbidity was limited to < 0.02 atomic units (AU) at 610 nm. The IC50 values (in µg/mL) were established to be 0.6 µg/mL for bakuchiol and 27 µg/mL for salicylic acid.
Collagenase inhibitory activity: The impact of bakuchiol on collagenase activity was measured with an enzyme kitc using quenched fluorescent gelatin and clostridium collagenase IV, a generic metalloproteinase. The test materials included aqueous solutions in the following concentrations: 1,000 µg/mL, 100 µg/mL, 10 µg/mL and 1 µg/mL and were made from 10 mg/mL stock in dimethyl sulfoxide (DMSO). Each was incubated in the presence of collagenase substrate-quenched fluorescin-linked gelatin and the proteolytic enzyme. Phenanthroline, a potent metalloprotease (MP) inhibitor, was used as positive control at 100 µg/mL. The kinetics of the release of the digested fluorescent gelatin were measured at excitation/emission wavelengths of 485/530 nm with a microfluorometerd.
Elastase inhibitory activity: Bakuchiol and retinol (50%)e were dissolved in DMSO at 10 mg/mL. The test materials were assayed at the following final concentrations: 25 ug/mL, 5 ug/mL and 1 ug/mL by incubation with pure human neutrophil elastasef and its substrateg, in vitro. The positive control was an elastase substrateh, and ultrapure water—i.e., both biologically pure and free of trace metals and dissolved organics, was the negative control. The proteolytic activity of elastase in the presence of the test materials was measured by following the generation of a chromophoric reaction product at 410 nm using microplate readerj; the experiment was performed in triplicate.
Anti-inflammatory activity: Cyclooxygenase (COX) inhibition by bakuchiol and salicylic acid was measured at 37°C by monitoring oxygen consumption using an oxygen electrode control unitk. The COX-1 and COX-2 IC50 for bakuchiol were found to be 14.7 µg/mL and 514 µg/mL, respectively.
Clinical study: Four formulations were clinically evaluated for their ability to treat acne-affected skin. The formulations included: 1% bakuchiol, 2% salicylic acid, 1% bakuchiol (and) 2% salicylic acid, and a placebo—i.e., with no active. Each formulation was tested on 15 volunteers with a total of 60 volunteers (including four drop-outs) having mild (< 10 comedones), moderate (10 to 25 comedones) or severe (> 25 comedones) acne. Prior to admittance to the study, each subject was examined by a dermatologist and their facial skin condition, including actual counts of non-inflammatory and inflammatory lesions, was recorded separately for the right and left sides of the face. In the case of female subjects, the date of onset of last menstruation was also recorded. Each product was applied twice daily in an amount sufficient to provide a light coating on the face and the percent reduction in acne was determined using the Global Acne Grading System.18 This system takes into account both non-inflammatory and inflammatory lesions. Non-inflammatory lesions included open comedones (blackheads) and closed comedones (white heads and un-inflamed nodules, sometimes called cysts); and inflammatory lesions included papules (small red bumps), pustules (white or yellow squeezable spots) and inflamed nodules (large red bumps).
Discussion and Results
As previously described, the enzyme 5-α-reductase converts testosterone to DHT, which binds to androgen receptors on the sebaceous glands and causes excessive oil production. Excess oil obstructs the skin pores, allowing bacterial growth that causes inflammation, infection and visible acne. Therefore, ideally, a product that either down-regulates the formation of 5-α-reductase synthesis or is an inhibitor of 5-α-reductase activity could be an effective solution. The described studied shows that bakuchiol is effective in down-regulating the formation of 5-α-reductase. At 10 µg/mL, both bakuchiol and retinoic acid showed approximately 40% reductions in 5-α-reductase expression, compared with the placebo (see Figure 2).
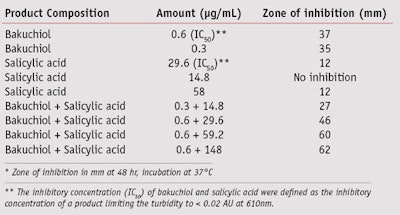
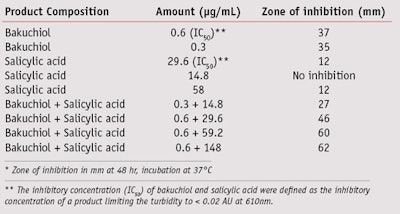
Broad antibacterial/antifungal activities are also desired for anti-acne treatments since affected skin has shown much higher levels of Staphylococcus and Candida.19, 20 The described study demonstrates that bakuchiol exhibits excellent P. acnes inhibitory activity, and it is very effective in inhibiting other microorganisms such as Staphylococcus and Candida. The minimum inhibitory concentrations (MIC, in µg/mL) of bakuchiol were 1 µg/mL for Staphylococcus aureus, 2 µg/mL for S. epidermidis and 1.5 µg/mL for Candida albicans. Further, while salicylic acid is a poor inhibitor of P. acnes, at certain concentrations, bakuchiol in combination with salicylic acid was found to be synergistic in inhibiting P. acnes (see Table 1). In comparison, a recent clinical study showed that retinoids such as retinoic acid and retinol did not exhibit any antibacterial activity, whereas retinaldehyde was an effective inhibitor of Staphylococcus and Candida.21
Literature also has reported that matrix metalloprotease (MMP) levels in acne-affected skin are much higher than in normal skin22, 23 and they play a predominant role in inflammatory matrix remodeling and hyperproliferative skin disorders. In addition, MMPs cause destruction of extracellular matrix proteins like collagen, fibronectin, laminin and elastin. Diminishing the presence of matrix-degrading enzymes in the acne lesion reduces imperfect repair of the skin and thus decreases scarring in acne-affected skin.
In relation, the described study determined that the collagenase inhibitory concentration 50% (IC50) for bakuchiol was ~0.1% w/w, whereas bakuchiol had a clear inhibitory activity at all concentrations tested against elastase. In contrast, the described study showed that retinol had no statistically significant elastase-inhibitory effect at all concentrations tested; the elastase inhibitory activity (IC50 value) for bakuchiol was found to be between 1 µg/mL and 5 µg/mL, but a trend toward reverse dose-response was observed. EI III completely blocked elastase activity, demonstrating the technical success of the experiment.
Inflammation is another severe problem in acne-affected skin. Unfortunately, few options are available to directly treat the inflammation that accompanies acne. However, the described studies show that bakuchiol has a moderate inhibitory activity against COX-2 (IC50 514 µg/mL) and a strong inhibitory activity against COX-1 (IC50 14.7 µg/mL). In addition, a large amount of nitric oxide (NO) production following the induction of inducible NO synthase (iNOS) gene has been implicated in the pathogenesis of inflammatory diseases. Literature has shown bakuchiol to be effective in inhibiting the expression of inducible nitric oxide synthase genes via the inactivation of nuclear transcription factor-κB in mouse leukaemic monocyte macrophage cell lines (RAW 264.7) macrophages.24 Interestingly, retinol was not found to be an iNOS inhibitor whereas retinoic acid was.25 Further, bakuchiol is a weak inhibitor of phospholipase A2 but dose-dependently inhibits the formation of leukotriene B4 and thromboxane B2.26
It should be noted that the oxidative breakdown of squalene and other skin lipids may not merely be a consequence of the acne process; it was suggested by Lorincz in 1965 that lipid peroxides might be directly acnegenic to the skin.27, 28 In a small, controlled pilot study (n = 15), Lorincz also found clinical success using topical alpha-tocopherol (0.05%) to treat acne after one month of evaluation by an independent examiner.27 Further support to the lipid peroxidation hypothesis came from Tappel, who reported in 1975 that lipid peroxidation is evident in acne and that localized free radical damage and peroxides might be involved in initiating damaging inflammatory reactions.29 Other investigators also reported that components of sebum, particularly squalene, show enhanced comedogenicity when oxidized.30 Squalene was shown to be highly sensitive to oxidation and researchers reported that both squalene and its oxidized metabolites are found at much higher levels in acne vs. healthy controls.28, 31
Another recent clinical study confirmed the role of reactive oxygen species in the inflammation of acne by determining the activity of antioxidant defense enzymes in leukocytes.32 Included in the study were 52 patients with papulopustular type acne vulgaris and 36 healthy controls, and the severity of the disease was examined by the Global Acne Grading System. The activities of superoxide dismutase (SOD), glutathione peroxidase and catalase enzymes as well as the level of thiobarbutric acid reactive substances (TBARS) were determined in leukocytes. The activities of SOD and glutathione peroxidase were found to be significantly decreased in the acne group. Catalase and TBARS levels were higher in patients than the control group, and only a poor correlation was detected between glutathione peroxidase activity and severity of the disease. Authors suggested that antioxidants be included in the acne treatment regimen.
The described study also showed that bakuchiol has broad-spectrum antioxidant activity and effectively quenches superoxide-, hydroxy-, peroxy-, peroxynitrile radicals and singlet oxygen non-radicals in addition to inhibiting lipid peroxidation (see Table 2). Further, the literature has shown bakuchiol to effectively reduce lipid peroxidation,33 protect mitochondria from NADPH-dependant and ascorbate-induced lipid peroxidation,34 and protect mitochondrial respiratory enzyme activities against both NADPH-dependant and dihydroxyfumarate-induced peroxidation injury.35 Squalene is particularly prone to photooxidation during sun exposure,36 and bakuchiol is expected to protect squalene and other skin lipids from oxidation due its excellent lipid peroxidation inhibitory activity (see Table 2).
Finally, the pilot clinical study demonstrated that bakuchiol effectively reduces acne and is more effective when combined with salicylic acid. The percent reduction in acne using the Global Acne Grading System14 is summarized in Table 3. Based on the results, formulations containing 1% bakuchiol (and) 2% salicylic acid showed a nearly 70% reduction in acne lesions and inflammation, as judged by the acne grading system. The next best results were with 1% bakuchiol, which reduced acne by a score of about 57%, whereas 2% salicylic acid only reduced acne by about 48%. As expected, the control group provided practically no improvement in the reduction of acne. None of the subjects observed or reported any adverse reaction using these formulated products. These results clearly show that bakuchiol is an effective ingredient (see Figures 3 and 4), especially when combined with an exfoliating agent like salicylic acid, for the treatment of acne.
Formulating With Bakuchiol
Bakuchiol is miscible with a wide variety of emollients including capric/caprylic triglycerides, C12–15 alkyl benzoates, and mineral oils; vegetable oils; and silicones such as dimethicones and cyclomethicones. To use bakuchiol, it must be dissolved in the appropriate hydrophobic emollient(s), then added to the formulation after making the emulsion. Solutions of bakuchiol may be added to formulations at a processing temperature of about 50°C or below. Alternately, it can be added directly to the oil phase. For preparation of anhydrous serums or transparent gels, silicone derivatives such as dimethicone, cyclomethicone, or dimethiconol (and) dimethicone crosspolymer may be used.
Contact with iron or copper compounds must be avoided as bakuchiol is a phenolic compound, which by nature, tends to become colored in the presence of metal ions, although the addition of a small amount of disodium EDTA, 0.05%, resolves this problem. The recommended use level of bakuchiol is from 0.5% to 1% w/w of finished formulation and an acidic pH (< 6.5) is necessary. Finally, bakuchiol was found to be compatible with both salicylic acid and benzoyl peroxide as evidenced from stable formulations containing these two anti-acne ingredients.
Conclusion
Phenol, 4-[1E, 3S)-3-ethenyl-3, 7-dimethyl-1, 6-octadienyl (bakuchiol), a meroterpene of plant origin, shows promise as a new agent that can complement and enhance the effectiveness of currently available anti-acne formulations. Bakuchiol is likely the only agent after retinoic acid shown to be effective against multiple pathophysiologic features of acne.
Further, bakuchiol has an excellent safety profile and was shown to be non-irritating and non-sensitizing based on human repeat insult patch testing (data not shown), has no photo- or hydrolytic stability issues, and thus can be used throughout the day. Topical formulations that include bakuchiol are likely to lead to further improvements in the way the industry treats skin infected by acne and beyond.
References
Send e-mail to [email protected].
1. JJ Leyden, New understandings of the pathogenesis of acne, J Am Acad Dermatol 32 S15–S25 (1995)
2. Acne—Drug Pipeline Analysis and Market Forecasts to 2016, available at www.researchandmarkets.com/research/3ac192/acne_drug_pipeli (Accessed May 6, 2011)
3. WJ Lee et al, Effect of dihydrotestosterone on the upregulation of inflammatory cytokines in cultured sebocytes, Arch Dermatol Res 302(6) 429–433 (2010)
4. Y Katsuta, T Iida, S Inomata and M Denda, Unsaturated fatty acids induce calcium influx into keratinocytes and cause abnormal differentiation of epidermis, J Invest Dermatol 124(5) 1008–1013 (2005)
5. M Bek-Thomsen, HB Lomholt and M Kilian, Acne is not associated with yet-uncultured bacteria, J Clin Microbiol 46 (10) 3355–3360 (2008)
7. I Kurokawa et al, New developments in our understanding of acne pathogenesis and treatment, Experimental Dermatology, 18 821–832 (2009)
8. EA Eady, M Gloor and JJ Leyden, Propionibacterium acnes resistance: A worldwide problem, Dermatology 206 54–56 (2003)
9. DM Thiboutot and HP Gollnick, Treatment considerations for inflammatory acne: Clinical evidence for adapalene 0.1% in combination therapies, J Drugs Dermatol 5(8) 785–794 (2006) 10. AL Zaenglein and DM Thiboutot, Expert Committee Recommendations for Acne Management, Pediatrics 118 1188–1199 (2006)
11. I KurokawaI et al, New developments in our understanding of acne pathogenesis and treatment, Exp Dermatol 18(10) 821–32 (2009)
12. R Kaul, Kinetics of the anti-staphylococcal activity of bakuchiol in vitro Arzneimittelforschung 26(4) 486–489 (1976)
13. H Katsura, RI Tsukiyama, A Suzuki and M Kobayashi, In vitro antimicrobial activities of bakuchiol against oral microorganisms, Antimicrob Agents Chemother 45(11) 3009–3013 (2001)
14. RK Chaudhuri, The miracle of retinol, SPC 23–24 (May 2010)
15. RK Chaudhuri, K Bojanowski and F Marchio, Retinol and retinol-like compounds in skin care, Expression Cosmetique 227–233 (2011)
16. US Patent 20090137534 and EP 2144590A1, Skin treatment composition and methods, RK Chaudhuri, assigned to Sytheon Ltd., (May 28, 2009; Jan 20, 2010)
17. RK Chaudhuri, R Sharma, KR Ranganathan, G Gutierrez and M Serrar, Is plant-derived meroterpene an effective replacement of retinoid for the treatment of acne-affected skin? proceedings of 25th IFSCC, Barcelona, Spain 3 65–70 (2008)
18. BM Burke and WJ Cunliffe, The assessment of Acne vulgaris—the Leeds technique, British J Dermatol 111 83–92 (1984)
19. S Nishijima, I Kurokawa, N Katoh and K Watanabe, The bacteriology of Acne vulgaris and antimicrobial susceptibility of Propionibacterium acnes and Staphylococcus epidermidis isolated from acne lesions, J Dermatol 5 318–323 (2000)
20. L Slobododnikova, D Kostalova, D Labudova and V Kettman, Antimicrobial activity of Mahonia aquifolium crude extract and its major isolated alkaloids, Phytother Res 18(8) 674–676 (2004)
21. M Pechere, L Germanier, G Siegenthaler, JC Pechere and JH Saurat, The antibacterial activity of topical retinoids: The case of retinaldehyde, Dermatol 205(2) 153–158 (2002)
22. E Papakonstantinou et al, Matrix metalloproteinases of epithelial origin in facial sebum of patients with acne and their regulation by isotretinoin, J Invest Dermatol 125 (4) 673–684 (2005)
23. S Kang, S Cho, JH Chung, C Hammerberg, GJ Fisher and JJ Voorhees, Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-κB and activator protein-1 in inflammatory acne lesions in vivo, Am J Pathol 166(6) 1691–1699 (2005)
24. HO Pae et al, Bakuchiol from Psoralea corylifolia inhibits the expression of inducible nitric oxide synthase gene via the inactivation of nuclear transcription factor-κB in RAW 264.7 macrophages, International Immunopharmacol 1(9–10) 1849–1855 (2001)
25. GS Oh et al, Inhibitory effect of retinoic acid on expression of inducible nitric oxide synthase gene in l929 cells, Immunopharmacol Immunotoxicol 23(3) 335–342 (2001)
26. ML Ferandiz, The effect of bakuchiol on leukocyte functions and some inflammatory responses in mice, J Pharm Pharmacol 48(9) 975–980 (1996)
27. AL Lorincz, Human skin lipids and their relation to skin diseases, Armed Services Technical Information Report, AD467008:1–12 (1965)
28. WP Bowe and AC Logan, Clinical implications of lipid peroxidation in Acne vulgaris: Old wine in new bottles, Lipids in Health and Disease, 9 141–151 (2010)
29. AL Tappel, Lipid peroxidation and fluorescent molecular damage to membranes, pathological aspects of cell membranes, J Exp Botany, 145–170 (1975)
30. OH Mills, M Porte and AM Kligman, Enhancement of comedogenic substances by UV radiation, Brit J Dermatol 98 145–150 (1978)
31. D Saint-Leger, A Bague, E Cohen and M Chivot, A possible role for squalene in the pathogenesis of acne. I. In vitro study of squalene oxidation, Brit J Dermatol 114 535–542 (1986)
32. PY Basak, F Gultekin and I Kilinc, The role of the antioxidative defense system in papulopustular acne, J Dermatol 28(3) 123–127 (2001)
33. A Adhikari et al, Antioxidant activity of bakuchiol: Experimental evidences and theoretical treatments on the possible involvement of the terpenoid chain, Chem Res Toxicol 16 1062–1069 (2003)
34. H Haraguchi, J Inoue, Y Tamura and K Mizutani, Inhibition of mitochondrial lipid peroxidation by bakuchiol, a meroterpene from Psolalea corylifolia, Planta Medica 66(6) 569–571 (2000).
35. HH Inouye, J Inoue, Y Tamura and K Mizutani, Antioxidative components of Psoralea corylifolia (Leguminosae), Phytotherapy Res 16(6) 539–544 (2002)
36. B Auffray, Protection against singlet oxygen, the main actor of sebum squalene peroxidation during sun exposure, using Commiphora myrrha essential oil, Internation J Cosmetic Sci 29(1) 23–29 (2007)