The extracellular matrix (ECM) is the structural backbone of many tissues, especially the skin, and represents a main target for cosmetic applications. ECM proteins are believed to play a pivotal role in cellular migration, proliferation and gene regulation during wound healing. Fragments from ECM constituents have been found capable of stimulating ECM biosynthesis to compensate for tissue destruction.1 Their mechanisms have been implicated in wound healing, skin aging and skin’s response to UV irradiation;2, 3 from this knowledge, new actives have evolved, as the authors describe here.
Building from the concept that ECM constituents stimulate ECM biosynthesis, bioinformatic methods were employed to identify highly repetitive amino acid motifs with inherent antiaging activities. Several dozens of tetrapeptides were found scattered across sequences of the major ECM macro- molecules.4 Ten peptides showed the desired effect of significantly increasing collagen protein in supernatant, thus verifying the underlying assumption that breakdown products of ECM proteins stimulate the ECM neosynthesis.
Of the ten peptides, the five most promising were subjected to further analysis including concomitant collagen determination in the supernatant, as well as gene expression analysis of the ECM marker genes: collagen (COL1A1), fibronectin (FN1) and hyaluronic acid synthetase (HAS1).
Collagen, being a dermal protein responsible for skin strength and elasticity, was examined since its degradation leads to wrinkles that accompany aging.5 Hyaluronic acid, one of the main components of the ECM, is a nonsulfated glycosaminoglycan that binds water, also ensuring the elasticity of the skin.6 Fibronectin, a glycoprotein that helps to create a cross-linked network within the ECM, was also of interest since it provides binding sites for other ECM components such as hyaluronic acid and collagen.7
In the end, one tetrapeptide, glycine-glutamic acid-lysine-glycine (GEKG, or INCI: tetrapeptide-21)a, was evaluated in vivo for effects on these genes.
Material and Methods
Human dermal fibroblasts (HDFs) prepared from neonatal foreskin were cultured for four days in a humidified 5% carbon dioxide atmosphere in Eagle’s minimal essential mediumb supplemented with: 5% fetal calf serumc, 0.1% l-glutamine, 2.5% sodium bicarbonate, and 1% streptomycin/amphotericin B, until they reached confluence. For the studies described here, only early passage fibroblasts (< 12) were used so as to avoid any changes in their original phenotype during subculture. Cells were kept in 6-well plates for culture.
For isolation of total RNA, kitsd were used according to manufacturer instructions. The RNA concentration was determined via photometric measuremente at 260/280. To avoid repeated free-thaw cycles for the prepared RNA for multiple experiments, aliquots of total RNA (100 ng) were applied for cDNA synthesis using a synthesis systemf for the reverse transcription step with random healers. For each gene, a specific primer pair was designedg based on the cDNA sequence published as indicated. For each gene expression determination, three independent experiments were performed and the mean value of these was calculated.
PCR reactions were carried out using a continuous fluorescence detection deviceh and softwarej. Each sample was analyzed twice, employing the universal protocol over 46 cycles. Detailed reaction conditions included: 10 min 94°C of hot-start taq polymerase activation, 20 sec 95°C denaturation, 20 sec 55°C annealing, and 30 sec 72°C extension. For comparison of relative expression in real time PCR control cells and treated cells, the 2 (-ΔΔC(T)) method was used.
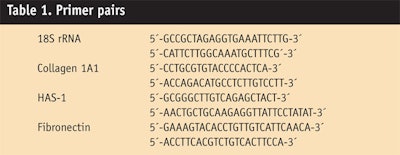
The tested peptides were applied at concentrations of 1 µg/mL for 24 hr to human dermal fibroblast cell cultures, and RNA was extracted to perform gene expression profiling. Induction of hyaluronic acid-synthase-1 and collagen was analyzed by real time PCR with the primer pairs shown in
Table 1. Since collagen production depends not only on stimulated gene expression, but also on a complex process of post-translational modification, researchers further quantified the collagen concentration in the fibroblast cell culture supernatants using a collagen assayk.8 All samples were incubated in the presence of β-aminopropionitrile (50 µg/mL) to increase the stability of the collagen.
In addition, an in vivo study was conducted with a panel of 60 volunteers divided into four groups. Four variations of a test cream were developed (see Formula 1), including a placebo cream without the active,
a positive control cream incorporating 10 ppm palmitoyl pentapeptide-4,2, 3 a cream with 10 ppm GEKG, and a cream with 100 ppm GEKG. The samples were applied to the inner forearms of subjects twice daily for eight weeks. Before and after eight weeks of application, skin volume and roughness were analyzed using a skin surface characterization devicem.
Results
Of all those tested, the most active peptide found had the sequence GEKG (see Figure 1). At a concentration of 1 μg/mL, the peptide increased the amount of secreted collagen protein in the supernatant approximately 2.5-fold. On the mRNA level, all three tested ECM marker genes were induced, resulting in a 2.5-fold increase of COL1A1 expression. In addition, HAS1 encoding for the hyaluronic acid was 5.7-fold, and the gene encoding for fibronectin was induced by 10.5-fold. The well-balanced induction of these important ECM constituents by the GEKG peptide suggests strong antiaging effects.
Although the palmitoyl pentapeptide-4 positive control showed a stronger induction in COL1A1 gene expression, the effect was not completely translated into collagen protein. This could be due to different gene induction kinetics. For instance, GEKG may cause a faster response on expression levels that is immediately translated into protein production, and that afterward diminishes to minor levels; whereas the positive control peptide could take longer to react to the stimulus, thus delaying the response.
The in vivo relevance of GEKG activity was additionally tested by a vehicle-controlled biopsy and elasticity study. After eight weeks’ application of a cream formulation containing50 ppm GEKG, both collagen gene expression and skin elasticity significantly increased, compared with the vehicle and untreated skin (data not shown but available).
Thus, to demonstrate the proposed antiaging effect of GEKG in vivo, a parameter “volume” measurement was taken of the previously mentioned 60 volunteers. This software-based method compares the distribution of gray scales in photographs taken of the volunteers’ skin before and after the eight-week application period. It calculates the theoretical amount of liquid that would be necessary to fill the wrinkles and generate a plain surface. A reduction of the parameter volume is interpreted as an overall improvement in skin structure resulting from the reduction of skin wrinkles in number and depth.
With an increasing concentration of GEKG, an increased reduction of the volume was observed. Compared with the positive control, a significant increase was obtained with100 ppm GEKG (see Figure 2a).
Besides parameter volume measurements, parameter roughness was assessed via the same skin texture analysis devicem.9,10 These roughness parameters originate from the DIN-parameters Ra–Rz, and describe the depth of fine and coarse wrinkles. R1–R5, for instance, describe the maximum and average amplitude of a surface structure, as well as the mean height level. In the case of GEKG in skin, Figure 2b again demonstrates a dose-dependent effect; increasing concentrations of GEKG increased the reduction of skin roughness. Compared with the positive control, 10 ppm GEKG showed comparable efficacy whereas 100 ppm GEKG doubled the effect.
Only 10 ppm of palmitoyl pentapeptide was tested, which is closer to its maximum suggested use level. GEKG at 10 ppm and 100 ppm translates to approximately 0.5–5.0% of the tetrapeptide-21 use concentration, which contains 2,000 ppm peptide.
Figure 3 shows the skin structure of one volunteer who applied the formulation containing 100 ppm GEKG for eight weeks. The pictures demonstrate an overall improvement of the skin structure; the wrinkles are less deep and less pronounced and the skin roughness is decreased.
Conclusion
Aging is associated with changes in the skin at all levels. For instance, degradation of key dermal-epidermal and dermal proteins occur, together with reduced epidermal proliferation and collagen and hyaluronic acid synthesis in the papillary dermis. Antiaging technologies must correct these deficiencies in order to induce skin regeneration to combat the resulting signs of aging.
The data presented in this study demonstrates that ECM-derived tetrapeptides have the potential to counterbalance the ECM degeneration observed during skin aging. In silico analysis identified approximately 30 abundant tetrapeptide motifs in ECM proteins, and in vitro analysis showed that 10 of these motifs can stimulate collagen synthesis.
GEKG was identified as a highly active tetrapeptide that is able to stimulate dermal repair and renewal mechanisms. An increased expression of ECM-synthesizing enzymes like hyaluronic acid synthethase, as well as expression and production of ECM proteins like collagen and fibronectin, was observed. Finally, in vivo data confirmed the efficacy of GEKG, leading to an improved skin structure and reduced wrinkles.
References
1. LR Robinson, NC Fitzgerald, DG Doughty, NC Dawes, CA Berge and DL Bissett, Topical palmitoyl pentapeptide provides improvement in photoaged human facial skin, Int J Cosm Sci 27(3) 155–160 (2005)
2. KT Tran, P Lamb and JS Deng, Matrikines and matricryptins: Implications for cutaneous cancers and skin repair, J Dermatol Sci Oct 40(1) 11–20 (2005)
3. KT Tran, L Griffith and A Wells, Extracellular matrix signaling through growth factor receptors during wound healing, Wound Repair Regen 12(3) 262–8 (May-Jun 2004)
4. US 20070299105, Peptide fragments for inducing synthesis of extracellular matrix proteins
5. M Yaar and BA Gilchrest, Photoaging: Mechanism, prevention and therapy, Br J Dermatol, 157(5) 874–87 (Nov 2007)
6. L Baumann, Skin aging and its treatment, J Pathol 211(2) 241–51 (Jan 2007)
7. M Larsen, VV Artym, JA Green andKM Yamada, The matrix reorganized: Extracellular matrix remodeling and integrin signalling, Curr Opin Cell Biol 18(5) 463–71 (Oct 2006)
8. VV Yurovsky, Tumor necrosis factor-related apoptosis-inducing ligand enhances collagen production by human lung fibroblasts, Am J Respir Cell Mol Biol 28(2) 225–31 (2003)
9. K De Paepe, JM Lagarde, Y Gall, D Roseeuw and V Rogiers, Microrelief of the skin using a light transmission method, Arch Dermatol Res 292(10) 500–10 (2000)
10. A Pagoni, Photo-aging and photodocumentation, Cosmet Toil 117(1) 39–46 (2002)