Ultraviolet A (UVA) radiation accounts for 95% of the total UV radiation reaching the earth’s surface and covers a wavelength range from 320 nm to 400 nm1—the longest UV wavelength. UVA can penetrate though the stratum corneum to the epidermis and dermis and stimulate tanning and pigmentation, as well as cause skin aging. Although UVB is more immediately damaging to the skin than UVA, it is now recognized that sunscreen products should protect against UVA as well as UVB.
While most existing sunscreen products provide good protection against UVB, leading dermatologists have voiced concern over the lack of UVA protection offered by current sun care products.2 In addition, recent publications by Draelos et al.3 and Murphy et al.4 have pointed out the importance of good UVA protection in the management of photodermatoses and prevention of skin cancer.
However, optimizing the UVA performance of sunscreens is challenging, particularly for global markets since UVA performance and labeling requirements differ from one region to another, some being more difficult to meet. Consequently, sunscreen manufacturers are finding it necessary to re-formulate products to enhance UVA efficacy while maintaining a high sun protection factor (SPF).
Test Methods and Labeling Criteria
In Europe: The European Commission (EC) Recommendation for testing and labeling sunscreen products5 provides guidelines for UVA protection, including:
1. The UVA protection factor (UVAPF) should be at least one third of the labeled SPF. According to the EC Recommendation, this requirement is justified because “scientific findings show that certain biological damage to the skin can be prevented and reduced” if this criterion is met. The in vivo persistent pigment darkening (PPD) method is the reference method for measuring the UVAPF, although in vitro methods can also be used. The European cosmetics association (COLIPA) published an in vitro method to measure UVAPF in 2007 and updated it in 2009;6 and
2. The critical wavelength should be at least 370 nm.
Although these are mere guidelines that are not legally enforceable, most of the industry has chosen to self-regulate to meet the above criteria since they are key requirements for sun protection formulations in Europe.
In the United States: The US Food and Drug Administration’s (FDA) Sunscreen Monograph has been in development for more than 30 years. While the Final Monograph published7 in 1999 made no mention of UVA protection requirements or testing, proposed amendments published8 in 2007 did include proposals for UVA labeling and test methods. Two test methods are proposed: the in vivo PPD method, to give the UVAPF, and an in vitro test to determine the UVA-I/UV ratio. This is the ratio between the mean absorbance in the UVA-I region (340–400 nm) and the mean absorbance across the whole UVA and UVB.
For each test, four performance categories are defined that are denoted by star ratings—from one to four stars. The proposals specify that both tests should be conducted, and that the labeled star rating should be the lower of the two results. Readers should note that these proposals have not yet been finalized and few manufacturers have implemented this labeling system thus far.
In Japan: The in vivo PPD method is used in Japan, with three categories for labeling purposes. These categories include: PA+ for 2 ≤ UVAPF < 4; PA++ for 4 ≤ UVAPF < 8; and PA+++ for UVAPF ≥ 8. This method also is used in a number of countries in the Far East.
In other regions: Australia uses a simple spectroscopic transmission test to determine whether a product can be labeled as broad-spectrum; however this is under review and is likely to change to the method currently being developed by the International Standards Organization (ISO). In addition, Latin America tends to follow European test protocols and labeling guidelines.
Formulating Strategies
Various formulation strategies exist to develop products that meet these UVA requirements, as are described here; each has advantages and disadvantages.
Combining organic UVA and UVB filters: This is the preferred formulating strategy for many formulators due to familiarity with organic filters as well as the variety of filters available. However, complex mixtures involving multiple filters are often required to obtain high SPF levels and provide good UVA performance while also meeting regulatory limits for the maximum allowable amount of each filter in a formulation.9 Such formulations are often expensive due to the high concentrations of UV filters required. In addition, some organic filters suffer from photo-instability,10 which is an important consideration since most UVA test methods now incorporate a pre-irradiation step. Finally, with varying regulatory restrictions for UVA versus UVB requirements in place, it is increasingly difficult to create global formulations.
Combining organic and inorganic UV filters: Combining organic and inorganic UV filters is the most common approach to achieving high SPF claims while meeting UVA requirements. Oftentimes synergies can be found between organic and inorganic filters, allowing high protection factors to be achieved with relatively low levels of actives. However, certain organic filters, e.g. avobenzone, can interact with the surface of titanium dioxide and impart a yellow discoloration to formulations. This can be avoided by optimizing the coating on the titanium dioxide (TiO2) or by incorporating the TiO2 into the water phase.
Also, global formulations of this type are often restricted by regulations; for example, the combination of TiO2 and/or zinc oxide (ZnO) with avobenzone currently is not permitted in the United States, although such combinations have been proposed. It remains to be seen whether these combinations will be approved when the FDA issues the finalized Sunscreen Monograph.
TiO2 and ZnO: Due to the mildness of inorganic UV filters, combinations of TiO2 and ZnO often are used in formulations for children and sensitive skin, and for “natural” sunscreens, where an inorganic only or chemical-free claim is desired; such claims are not permitted in the United States but do appear in some parts of the world. However, high SPF formulations require high levels of ZnO in order to meet the European recommendation for UVA protection. This is because ZnO has a lower refractive index and hence lower efficacy than TiO2. This can lead to unacceptable aesthetic properties on skin such as whitening. In addition, intellectual property restrictions in certain countries, which limit the choices of ZnO grades allowed, together with the fact that ZnO is not yet on the approved UV filters list in Europe further restrict the flexibility of this TiO2 and ZnO combination.
Thus, to avoid many of the issues described above, the authors aimed to meet UVA requirements by using TiO2 as the sole active. However, most existing grades of TiO2 attenuate primarily in the UVB range, and while they provide high SPF efficacy, when used alone, they do not provide enough UVA protection to meet the European UVA criteria. Therefore, a new form of TiO2 was developed.
Tuning TiO2 for Enhanced UVA Protection
It is well-known that increasing the particle size of TiO2 increases the UVA attenuation of sunscreen formulations. Larger particle-sized TiO2 produced for sunscreens is commonly manufactured by high temperature calcination to change the crystal phase from anatase to rutile. Unfortunately, the temperature used to carry out this phase transformation produces powders with large portions of oversized particles. This results in the extensive scattering of visible light, which in turn gives products with excessive visible whitening.
However, by using a carefully controlled precipitation process, it is possible to prepare TiO2 nuclei with a narrow particle size distribution that are already in a rutile form, and thus tune the particle growth stage to balance the levels of UVA and UVB attenuation without excessive visible whitening. The enhanced UVA protection powder produced will be referred to herein as enhanced UVA TiO2. To optimize the efficacy of the enhanced UVA TiO2 powder, two dispersions were developeda. Two cosmetic oils were selected as carrier fluids: C12-15 alkyl benzoate, a common oil used in sun care products to solubilize organic UV absorbers; and caprylic/capric triglyceride, which is a naturally derived carrier. The latter of the two was chosen to offer a natural sun care formulation claim.
Materials and Methods
Dispersions were produced by milling techniques and analyzed using the methods described below before incorporating them into test formulas.
UV and visible radiation profiles: UV/visible (UV/Vis) performance was tested by a spectrophotometerb after diluting the oil-based dispersion in cyclohexane. Figure 1 shows the increase in UVA attenuation given by the enhanced UVA TiO2, compared with a conventional sunscreen grade TiO2. Careful control of the particle growth process therefore resulted in enhanced UVA attenuation as well as retention of UVB performance and a moderate whitening effect on skin.
Particle size: The particle sizes of the enhanced UVA TiO2 powder and conventional grade TiO2 were compared using an x-ray centrifuge methodc (see Figure 2). The curve for the conventional grade shows a broad tail of large particles, which leads to excessive whitening in formulation. Since the number of oversized particles in the enhanced grade was significantly reduced, this improved the aesthetic properties while maintaining excellent UVA attenuation. The mean particle size for the enhanced UVA TiO2 was 145 nm whereas the conventional grade measured 70 nm.
Coating: Although the enhanced UVA TiO2 described is designed to function as a stand-alone UV filter, it can also be used in combination with organic filters. So as to avoid the challenges of a combined system described previously, an effective coating is necessary. Therefore, a coating consisting of alumina and stearic acid was applied to the enhanced UVA TiO2 after production of the particles.
The integrity of the coating on the enhanced TiO2 particles was then tested using a method that indirectly measures the photochemical reduction of Ti4+.11 Upon exposure to UV light, electrons promoted from the valence to the conduction band of the TiO2 lattice leave positively charged holes in the valence band. Electrons and holes that reach the surface of uncoated TiO2 are free to react with surface adsorbed species such as oxygen and hydroxyl ions, which can lead to the formation of radicals. By providing an effective surface coating, the electrons and holes cannot react with surface adsorbed species. In addition, an effective coating inhibits the reduction of Ti4+ (colorless) to Ti3+ (purple) in the absence of oxygen, a process known as photogreying. This particular reduction process is used as a basis to test the quality of coating on the surface of TiO2 particles.
A sample of TiO2 dispersed in oil is exposed to UV light. Photogenerated electrons reduce Ti4+ ions, and the sample suffers from a gray discoloration as the concentration of Ti3+ increases. This gray discoloration is detected by means of a colorimeter measuring ΔL, the photogreying index, and compared with a standard material that is analyzed alongside the test sample.
Five samples of commercially available TiO2 were tested for photogreying using this process and compared with the results from a dispersion containing the enhanced UVA TiO2. Figure 3 shows that the enhanced UVA TiO2 sample had a low photogreying index, indicating that the particles are efficiently coated. This well-coated surface improves the dispersion properties of the particles12 and minimizes interaction of the TiO2 with other cosmetic ingredients in formulations.
Formulating with Enhanced UVA TiO2
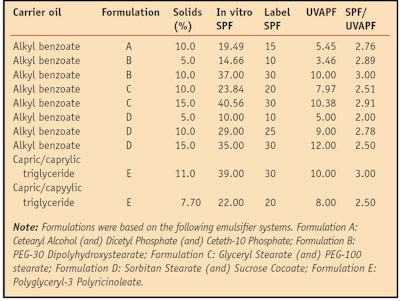
The two oil-based dispersions of enhanced UVA TiO2 were then incorporated into o/w and w/o sunscreen formulations at varying levels and the in vitro SPF values of the formulas were measuredd (see Table 1). The UVAPF also was measured in vitro using the COLIPA protocol.6
SPF values for the formulations meeting the EC 1/3 UVAPF requirement ranged from 10 to 30; Table 1 details the SPF, UVAPF and SPF/UVAPF ratio for these formulations. All formulations passed the European guidelines for UVA protection, indicating that the dispersions showed reliable performance. While w/o emulsions (formulas B and E) tended to provide higher SPF efficacy than o/w emulsions, there was no inherent difference in efficacy between the two dispersions; Figure 4 shows the SPF results for both dispersions used in the same basic formulation at various solids levels. An approximately linear increase in SPF was observed with TiO2 loadings of up to 15% solids. The data in Figure 4 and Table 1 indicate that every 1% solids provides approximately 2–3 SPF units.
Critical wavelength: According to the EC requirements for UVA protection, sun protection products must have a critical wavelength that is greater than 370 nm. Figure 5 shows the performance of a range of commercially available TiO2 and ZnO products in formulations. It should be noted that not all TiO2 products developed passed the critical wavelength test, and even some of the ZnO-containing formulations, which may be expected to pass comfortably, did not meet the 370 nm requirement.
Other UVA criteria: As indicated by Table 1, use of the enhanced UVA TiO2 at 10% solids was typically sufficient to achieve a UVAPF of at least 8, which would give a PA+++ rating on the Japanese labeling system. The in vitro UVA-I/UV values also were calculated for the formulations in Table 1 and these values ranged from 0.77 to 0.83. Such a result, together with a UVAPF of at least 8, would be sufficient to achieve a three-star rating under the FDA’s proposed system, should this eventually be adopted.
In vivo SPF tests: The SPF performance of three formulas derived from combinations shown in Table 1 were also confirmed in vivo using five panelists (see Formula 1, Formula 2 and Formula 3). The three formulations were tested according to the International Method13 and the results were found to be similar to those obtained in vitro.
Conclusion
The enhanced UVA TiO2 dispersion described here was shown to offer enhanced UVA attenuation while maintaining the high SPF efficacy typically seen with TiO2. This was achieved by producing optimized particles of TiO2 to boost UVA attenuation yet maintain high UVB performance. The number of oversized particles produced during the manufacturing process was reduced in order to moderate the whitening of the product on skin. In addition, oil-based dispersions of the active ingredient formulated into o/w and w/o emulsions as the sole active produced SPF 10 to SPF 30 sunscreens that met the EC guidelines for UVA protection, which would also be expected to produce high ratings on the Japanese and proposed US labeling systems for UVA.
This new grade of TiO2 will enable formulators to make broad-spectrum sunscreen formulations that meet various UVA testing/labeling requirements around the world using a single active. This potentially simplifies the formulation development process and hence shortens development time while enabling formulators to create products that meet current market trends.
References
- CG Nelson, Photoprotection, in Sunscreens: Regulations and Commercial Development, 3rd ed, NA Shaath, ed, Taylor and Francis, New York (2005) pp 19–43
- SQ Wang, The sunscreen race, Happi 47 (1) 38–40 (2010)
- ZD Draelos et al, Sunscreens and photodermatoses, in A Clinical Guide to Sunscreens and Photoprotection, HW Lim and ZD Draelos, eds, Informa Healthcare, New York (2009) pp 83–88
- GM Murphy and JLM Hawk: Sunscreens and photocarcinogenesis in A Clinical Guide to Sunscreens and Photoprotection, HW Lim and ZD Draelos, eds, Informa Healthcare, New York (2009) pp 89–99
- European Commission recommendation of Sep 22, 2006, on the efficacy of sunscreen products and the claims made relating thereto; Official Journal of the European Union L 265/39–43
- COLIPA Method for in vitro determination of UVA protection, 2009, available at www.colipa.eu/publications-colipa-the-european-cosmetic-cosmetics-association/guidelines.html?view=item&id=33 (accessed Jul 5, 2010)
- Sunscreen drug products for over-the-counter human use, Final monograph, Fed Reg 64 27666–27693 (May 21, 1999)
- Sunscreen drug products for over-the-counter human use: Proposed amendment of final monograph, proposed rule, Fed Reg 72 49070–49122 (Aug 27, 2007)
- NA Shaath, The Encyclopedia of Ultraviolet Filters, Allured Business Media, Carol Stream, IL (2007)
- CA Bonda, The photostability of organic sunscreen actives: A review, in Sunscreens: Regulations and Commercial Development, 3rd ed, NA Shaath, ed, Taylor and Francis, New York (2005) pp 321–349
- TA Egerton, LM Kessell, IR Tooley and L Wang, Photogreying of TiO2 nanoparticles, J of Nanoparticle Research 9 2 251–260 (2007)
- TA Egerton, The modification of fine powders by inorganic coatings, KONA Powder and Particle, No. 16 (1998) pp 46–58
- Mike Brown, SPF testing in Europe: The international SPF test method, in Sunscreens: Regulations and Commercial Development, 3rd ed, NA Shaath, ed, Taylor and Francis, New York (2005) pp 779–806