*Modified with permission from Archives of Dermatological Research, Springer
Editor’s note: This column is the first in a series on the relationship between substantivity and skin care products. This first part provides background on the role of substantivity and its safety considerations. Future installments will discuss its use in personal care.
Skin care and cosmetic formulators intuitively have a sense as to when a given product benefits from remaining on the skin, lips, nails and hair for prolonged periods. This series encapsules our knowledge on this aspect of formulation—substantivity.
A primary function of the skin barrier is attributed to the stratum corneum (SC), which protects us from mechanical insults, UV light, pathogenic microorganisms, chemical compounds and other percutaneous penetrants. While an effective barrier against percutaneous absorption of chemicals, the SC is also a major route by which chemicals may enter the body. Once absorbed into skin, penetrants may cause local reactions or enter the circulation to produce systemic therapeutic or toxic effects.
Ngo, expanding on Wester, delineated 15 factors that influence the ability of chemicals to penetrate the skin, although there are presumably more, demonstrating the complexities of this process.1, 2 Drug-related factors include: vehicle, molecular weight, lipid and water solubility, and other chemicals that may serve as penetration enhancers. Exposure-related factors include: dose, formulation, duration, frequency, use of protective equipment and climate (heat and humidity). Finally, skin-related factors include: blood flow, pH, skin thickness, hair and pore density, and content and structure of proteins and lipids.
Substantivity, a term adapted from the textile dye industry, is yet another factor; it allows penetrants to remain in the skin for many days. Although binding to viable epidermis and dermis has been noted, substantivity describes chemical adherence mainly to the stratum corneum.3 This SC adsorption allows for chemicals to enact their effects while overcoming abundant sweating, water immersion and abrasive rubbing. Therefore, substantivity is an especially important factor in the development of various drugs that target the SC.
Since many skin pathologies involve the stratum corneum (e.g., fungal infections, acne vulgaris, rosacea, atopic dermatitis and irritant dermatitis) and require the multiple dosing of topicals, understanding substantivity mechanisms may provide insight for topical dosing strategies, as well as longer-acting topical medications—possibly even a single dose of treatment agents that previously required multiple doses. Substantivity is also of importance in the development of other consumer products that necessitate adherence to skin, including sunscreens and insect repellents.
While adsorption of chemicals to the SC may enable water and rub resistance, it may be the strength of the chemical bond between penetrants and molecules of the horny layer—presumably keratin proteins—that dictates SC adherence. The ability of chemicals to partition from the SC and subsequently penetrate through skin, as well as cell turnover, hampers SC adherence (substantivity). Thus, highly substantive substances may induce delayed percutaneous penetration, reducing the risk of systemic toxicity.
However, excessive substantivity may play a role in the adverse effects mentioned previously (irritation, allergic reactions and systemic toxicity), since substantive materials allow for the accumulation of harmful penetrants. Continued research in this area may offer insight into dermatotoxicology and dermatopharmacology; studies related to hexachlorophene provide insight.
Hexachlorophene: A Case Study
In the 1960s and early 1970s, hexachlorophene was estimated to be in thousands of American skin care and cosmetics. This section explains its rise and fall, as well as lessons learned from this human calamity.
Retention of an antimicrobial agent in the SC is a desirable property for aversion of pathogen colonization. Substantive antiseptic agents, which interact with the horny layer to create a reservoir effect, exert antimicrobial activity for several days beyond the last application. Hexachlorophene is an example of such a substantive antimicrobial. This material was discovered and popularized by Gump when he found the compound was capable of retaining its antiseptic properties in soap.4 Subsequently, hexachlorophene became a standard component of innumerable kinds of consumer goods and medical formulations.
Around the same time, Staphylococci replaced Streptococci as a chief cause of infections in newborn nurseries.5 Thus, when Farquharson et al. reported on the control of an outbreak of Staphylococcal skin infections in nurseries utilizing a hexachlorophene-based bath routine, other studies soon discerned the advantages of hexachlorophene bathing to control Staphylococcal skin outbreaks in nurseries, as well as in reducing the incidence of Staphylococcal skin colonization in newborns.6-8
Substantivity is important in the development of consumer products that necessitate adherence to skin, including sunscreens and insect repellents.
Farquharson et al. also showed a drastic drop in cases of impetigo after the implementation of hexachlorophene-containing baths 24 hr after delivery and every other day thereafter.7 Due to a shortage of nursing staff in September 1950, though, the hexachlorophene bathing routine was amended to 24 hr after delivery and every third day thereafter instead of every second day. Within one week, five new cases of impetigo arose. When every-other-day bathing resumed, cases of impetigo ceased to recur. This suggests that the bacteriostatic film of hexachlorophene adheres to infants’ skin in clinically effective amounts for 48 hr.
Baldwin et al. reported similar results: lower incidents of Staphylococcal skin colonization in infants bathed with a liquid detergent containing hexachlorophene, compared with infants who received “dry” skin care.6 The incidence of nasal colonization by Staphylococci was 30% with hexachlorophene washing, but rose as high as 100% when washing was discontinued. Gluck and Wood also found a lower incidence of nasal colonization by Staphylococci in infants washed with hexachlorophene-containing preparations twice after birth and every day thereafter, compared with control, “unwashed” infants.8 Altogether, 51% of 500 control “unwashed” infants were positive for Staphylococcal colonization, while only 3% of 965 “washed” infants were positive for Staphylococcal colonization.
Gluck and Wood argued that the efficacy of hexachlorophene depended on continued contact with susceptible pathogenic microorganisms.8 Frequent application allowed for the substantive chemical to accumulate in adequate amounts on skin, thereby achieving adequate antibacterial activity. Studies on the efficacy of surgical hand-scrubbing supported this argument, as multiple washes with hexachlorophene proved far more effective in ridding skin of bacteria compared with a single wash.9 Furthermore, studies utilizing radioactive hexachlorophene showed just how substantive the compound was—a plateau in accumulation in skin was achieved within three or four washes.10
By the early 1970s, hexachlorophene had been used extensively for more than 20 years, and reviews of its toxicity began to surface.11, 12 The first report of hexachlorophene toxicity was in 1959 by Herter, who reported excoriations and convulsions in a newborn infant after four days of repeated application of 3% hexachlorophene lotion without subsequent rinsing.13
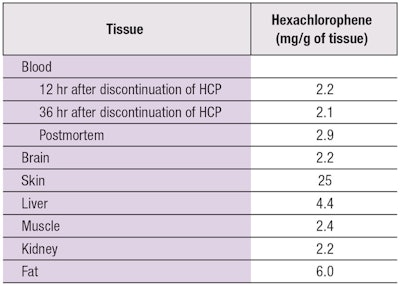
Later, Larson noted a correlation between hexachlorophene use and the encephalopathy observed in burn patients.14 He reported eight burn patients treated with topical hexachlorophene who developed seizures, diplopia, lower extremity weakness, nausea, vomiting and irritability. Incidence of convulsions in burn patients decreased upon cessation of hexachlorophene use.15 Chilcote et al. reported a similar finding in a 10-year-old boy who sustained a 25% partial-thickness burn and was treated with topical applications of diluted 3% hexachlorophene.16 The patient initially did well, but soon developed hyperthermia, lower extremity weakness and encephalopathy. Autopsy revealed cerebral edema and tissue with high hexachlorophene levels (see Table 1).
According to Carroll et al., hexachlorophene is absorbed through intact and damaged skin. Burns increased hexachlorophene absorption by a factor of 2.5 in rats.17 It is now widely accepted that most methods of damage (mechanical, chemical, biocidal and clinical disease) result in some enhancement of absorption.18, 19 However, in the study of Carroll and associates, absorption returned to baseline after 24 hr, suggesting excessive skin exposure and substantive properties, rather than altered permeability, were likely the principle players in toxicity.
Excessive substantivity may play a role in adverse effects, since substantive materials allow for the accumulation of harmful penetrants.
Manowitz and Johnston also illustrated the substantive properties of hexachlorophene.20 They noted that hexachlorophene was found on skin after soaking in a bath containing low concentrations of the chemical, and although a shower rinsed off a great amount of hexachlorophene, a residue remained post-cleaning with alcohol after the shower.
The most dramatic occurrence of hexachlorophene toxicity was in 1972 in France, where 18 children were accidentally exposed to 6% hexachlorophene in talc powder.21 The children (13 boys and 5 girls), between 3 months and 3 years of age, presented with neurological features including altered consciousness, encephalopathy, tremors, ocular jerks, mydriasis and sixth-nerve palsy. Twelve were under one year of age. Four patients died; eight developed spinal cord damage; and two became paraplegic.
After such a devastating event, and with substantial research of hexachlorophene toxicity with animal studies, the U.S. Food and Drug Administration banned all nonprescription uses of the chemical in 1972, only allowing for use as a surgical scrub for health care workers and low concentrations as a preservative. Hexachlorophene was also largely removed from the U.S. cosmetics market while other countries followed suit. This calamity brought the percutaneous penetration of skin care and cosmetic products to the forefront.
References
- Ngo, M. A., and Maibach, H. I. (2012). 15 Factors of Percutaneous Penetration of Pesticides. In Knaak, J. B., Timchalk, C., and Tornero-Velez, R. (eds): Parameters for Pesticide QSAR and PBPK/PD Models for Human Risk Assessment (67-86). Danvers, MA U.S.: Oxford Univ Press.
- Wester, R. C., and Maibach, H. I. (1983). Cutaneous pharmacokinetics: 10 steps to percutaneous absorption. Drug Metabolism Reviews, 14(2),169-205.
- Menczel, E., and Maibach, H. I. (1970). In vitro human percutaneous penetration of benzyl alcohol and testosterone: epidermal-dermal retention. J Invest Dermatol, 54(5), 386-394.
- Gump, W. S. (1945). Development of a germicidal soap. Soap and Sanitary Chemicals, 1, 36-39 and 50-85.
- Williams, R. E. O., Blowers, R., Garrod, L. P., Shooter, R. A. (1966). Hospital Infection: Causes and Prevention. London: Lloyd-Luke Ltd.
- Baldwin, J. N., Rheins, M. S., Sylvester, R. F., Jr., and Shaffer, T. E. (1957). Staphylococcal infections in newborn infants. AMA J Dis Child, 94(2), 107-116.
- Farquharson, C. D., Penny, S. F., Edwards, H. E., and Barr, E. (1952). The control of staphylococcal skin infections in the nursery. Can Med Assoc J, 67(3), 247-249.
- Gluck, L., and Wood, H. F. (1961). Effect of an antiseptic skin-care regimen in reducing in reducing staphylococcal colonization in newborn infants. N Engl J Med, 265(24), 1177-1181.
- Havens, I., Benham, R. S., and Clark, D. E. (1957). Hexachlorophene in the surgical scrub. Am J Med Technol, 23(2), 76-86.
- Shemano, I., and Nickerson, M. (1954). Cutaneous accumulation and retention of hexachlorophene-C14 (G-11). Fed Proc, 13, 404.
- Gump, W. S. (1969). Toxicological properties of hexachlorophene. J Soc Cosmet Chem, 20, 173-184.
- Kimbrough, R. D. (1971). Review of the toxicity of hexachlorophene. Arch Environ Health, 23(2), 119-122.
- Herter, W. B. (1959). Hexachlorophene poisoning. Kaiser Foundation M Bull, 7, 228
- Larson, D. L. (1968) Studies show hexachlorophene causes burn syndrome. Hospitals J Amer Hosp Assoc, 42, 63-64.
- Larson, D. L., Abston, S., and Bachmann, R. C. (1971). Hexachlorophene absorption from the burn wound. In Research in Burns (619). Stuttgart, West Germany: Hans Huber Publishers.
- Chilcote, R., Curley, A., Loughlin, H. H., and Jupin, J. A. (1977). Hexachlorophene storage in a burn patient associated with encephalopathy. Pediatrics, 59(3), 457-459.