Effective preservation clearly is important to inhibit the growth of microorganisms, e.g., bacteria, fungi and yeast, that could potentially harm consumers and/or cause cosmetics and personal care products to spoil.1, 2 However, as most formulators know, consumers have become more discriminating over the perceived human health and environmental effects of the ingredients in the products they use.
Preservatives in general, and parabens in particular, have received considerable scrutiny by not only consumers, but also ingredient suppliers, manufacturers, retailers, regulators and others. In response, this third article in our series on preservatives3, 4 summarizes the antimicrobial efficacy, human health and environmental data demonstrating the safety and benefits of this important class of preservatives.
Parabens in Action
Parabens refers to a group of esters of p-hydroxybenzoic acid whose general chemical structure is shown in Figure 1. The R in this figure refers to the carbon side-chain whose length differs among the different types of parabens, such as methyl, ethyl, propyl and butyl, commonly used as preservatives.
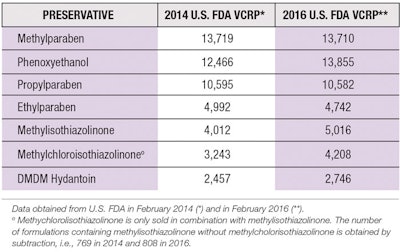
Parabens were first introduced in 1923 and have become some of the most commonly used preservatives in cosmetics, pharmaceuticals and foods.5, 6 Recent data from the U.S. Food and Drug Administration’s (FDA) Voluntary Cosmetic Reporting Program (VCRP) shows they continue to be among the most widely used preservatives in personal care products (see Table 1).
The reported range or spectrum of microbiological efficacy for parabens includes both bacteria and fungi.7-9 Due to a complexity of factors including water and lipid solubilities/partitioning, efficacy, propensity for microbial adaptation and even biodegradation by contaminants, paraben esters typically are used in combination with other preservatives.9, 10 Longer-chain esters (propyl and butyl parabens) primarily target yeasts and molds, while shorter-chain parabens (methyl and ethyl) are most effective against Gram-positive bacteria and, to a limited extent, Gram-negative bacteria.10 Thus, as stated, largely due to their limited efficacy against Gram-negative bacteria, parabens often are used in combination with other types of preservatives to establish a broader spectrum of antimicrobial efficacy. In practice, such systems are composed of parabens and formaldehyde-releasers or phenoxyethanol, and often facilitated with a chelator such as ethylenediaminetetraacetic acid (EDTA).10-13
Parabens are most commonly used in emulsion-based personal care products such as creams and lotions.8, 9 Though nominally effective across a broad pH range, parabens are most effective in acidic emulsions.8-10 In contrast, products composed of a high percentage of water, such as shampoos and conditioners, are primarily subject to contamination with Gram-negative bacteria. Therefore, parabens are less frequently used to preserve such formulas.8-10
Processes for manufacturing emulsions typically incorporate parabens dissolved in the heated water phase before emulsion formation.14 This approach is necessary to minimize the establishment or partitioning of preservatives in the lipid phase of the emulsion, where their efficacy is greatly mitigated. Standardized lab tests have been established to determine the efficacy of preservatives in personal care products,15 which have been correlated with consumer use testing.16
Due to a complexity of factors and properties, paraben esters typically are used in combination with other preservatives.
Safety Assessments: The Bottom Line
The safety profile of parabens has extensively been investigated by global regulatory bodies, academics and industry. Studies of parabens have included pre-clinical assessments of endpoints including acute and repeat dose toxicity, genotoxicity, reproductive toxicity, developmental toxicity, dermal irritation and sensitization, skin penetration and toxicokinetics. Clinical studies have evaluated their potential for irritation, sensitization and phototoxicity.
Parabens are not toxic at the concentrations used in personal care products. They are not genotoxic or carcinogenic, and not teratogenic. Parabens are readily excreted in urine and do not accumulate in tissues. The ester linkage appears to be readily hydrolyzed, resulting in p-hydroxybenzoic acid as the common metabolite of all parabens. Skin esterases are known to metabolize parabens following dermal exposure, while hydrolysis by esterases during first-pass metabolism has been demonstrated following oral route exposure.17-20
In its first review of parabens in 1984, the Cosmetic Ingredient Review (CIR) Expert Panel concluded, “Methylparaben, ethylparaben, propylparaben and butylparaben are safe as cosmetic ingredients in the present practices of use.”17 The safety of isopropylparaben and isobutylparaben were reviewed later, also resulting in a conclusion of “safe as cosmetic ingredients in the present practices of use.”18 Data used to reach the conclusion of safety included studies on iso-parabens as well as studies on straight chain parabens, which are considered relevant due to the similarity of the paraben preservatives and their common metabolite, p-hydroxybenzoic acid. In 2008, after reviews of new data, the CIR again concluded parabens are safe as cosmetic ingredients in current practices of use.19
In 2005, in the European Union, the former Scientific Committee on Consumer Products (SCCP, now called the Scientific Committee for Consumer Safety or SCCS) affirmed the safety of methylparaben and ethylparaben, concluding the two parabens could be safely used at levels up to 0.4%.21 However, the same opinion concluded more data was needed to evaluate propyl-, butyl-, isopropyl- and isobutylparaben. Thus, additional data was submitted, and subsequent opinions were issued.22, 23
In 2010, the SCCS concluded the sum of the individual concentrations of propyl- and butylparaben should not exceed 0.19% based on a “conservative choice for the calculation of the Margin-of-Safety (MoS) of butyl- and propylparaben.”24 The issue of concern related to reports of toxicity in juvenile male rats; specifically, decreased sperm counts and testosterone levels.25-27 However, these results were not replicated in larger studies conducted under Good Laboratory Practice (GLP) conditions and evaluating the same endpoints.28, 29 The SCCS also concluded the available data was not adequate to evaluate the human risk of isopropyl- or isobutylparaben.30
The issue of endocrine modulation (estrogenicity) also deserves mention.31-33 In vitro, parabens have shown weak estrogenic activity that increases with an increasing length and branching of the alkyl side chains. Their potency is 1,000 to 1,000,000-fold less than 17 β-estradiol.34 In vivo, parabens show weak estrogenic activity in some studies, but again with very low potency (approximately 10-5 to 10-6 that of estradiol). The common metabolite, p-hydroxybenzoic acid, shows no estrogenic activity when tested either in vitro or in vivo.35 A report36 purportedly linked parabens in underarm cosmetics to breast cancer; this has received widespread attention but upon further review, “no evidence of demonstrable risk” was identified.34
Environmental Assessments
From a lifecycle perspective, parabens used in cosmetic formulas generally are biodegradable, with standard and non-standard test data showing high removal rates (88.4-99.9%).37-39 Where biodegradation data is not available, U.S. Environmental Protection Agency (EPA) models (BIOWIN) predict parabens will be biodegraded. As such, parabens are likely to be removed during wastewater treatment.
Partitioning values suggest parabens are likely to be found in the aquatic environment, although not bioaccumulating significantly. In light of this data, the aquatic toxicity of parabens is of most relevance. Lower aquatic toxicity values (0.2-2.0 mg/L) have been reported in standard chronic (long-term) testing. However, due to the fact that parabens are rapidly degraded, they are not expected to pose significant environmental risks. The more relevant standard acute (short-term) ecotoxicity values are well above the EPA level of no concern (10 mg/L).37, 38 Indeed Yamamoto and co-workers reported that risk ratios for a range of widely used parabens are well within safety limits.39
Parabens are rapidly degraded, thus they are not expected to pose any environmental risks.
Conclusions
Parabens are important preservatives that demonstrate safe human and environmental profiles. This supports their effective use in cosmetics, pharmaceuticals and foods, although the industry has been pressured by consumer preferences and responded by providing alternative preservatives for some products. Regardless of the preservation system used, today’s personal care products meet rigorous standards for safety and antimicrobial efficacy.
References
- LA Wilson and DG Ahearn, Pseudomonas induced corneal ulcers associated with contaminated eye mascara, Am J Ophthalmol 84 112-119 (1977)
- A Madania et al, Serratia marcescens-contaminated baby shampoo causing an outbreak among newborns at King Abdulaziz University Hospital Jeddah, Saudi Arabia, J Hosp Infect 78 16–19 (2011)
- Cosmeticsandtoiletries.com/formulating/function/preservatives/premium-The-Importance-of-Formaldehyde-Donor-Preservatives-in-Personal-Care-Products-215078811.html (Accessed May 5, 2017)
- Cosmeticsandtoiletries.com/research/chemistry/Phenoxyethanol-as-a-Safe-and-Important-Preservative-in-Personal-Carepremium-256198651.html (Accessed May 5, 2017)
- E Luck and M Jager, Antimicrobial Food Additives: Characteristics, Uses, Effects, 2nd edn, Springer, New York (1997)
- Cosmeticsandtoiletries.com/regulatory/region/northamerica/Frequency-of-Preservative-Use-Update-Through-2014-367684531.html (Accessed May 5, 2017)
- Americanpharmaceuticalreview.com/Featured-Articles/38886-Antimicrobial-Preservatives-Part-One-Choosing-a-Preservative-System/ (Accessed May 5, 2017)
- Americanpharmaceuticalreview.com/Featured-Articles/38885-Antimicrobial-Preservatives-Part-Two-Choosing-a-Preservative/ (Accessed May 16, 2017)
- PA Geis, Preservation strategies, in Cosmetic Microbiology: A Practical Handbook, 2nd edn, PA Geis, ed, CRC Press, New York (2006) pp 163-180
- TE Haag and DF Loncrini, Esters of para-hydroxybenzoic acid, in Cosmetic and Drug Preservation, JJ Kabata, ed, Marcel-Dekker, New York (1984) pp 63-77
- RME Richards and RJ McBride, Phenylethanol enhancement of preservatives used in ophthalmic preparations, J Pharm Pharma 23 141S-146S (1971)
- WE Rosen, PA Berke, T Matzin and AF Peterson, Preservation of cosmetic lotions with imidazolidinyl urea plus parabens, J Soc Cosmet Chem 28 83-87 (1977)
- SP Denyer, BW Hugo and VD Harding, Synergy in preservative combinations, Intl J Pharmaceutics 25 245-253 (1985)
- NK Patel and JM Romanowski, Heterogeneous systems II: Influence of partitioning and molecular interactions on in vitro biologic activity of preservatives in emulsions, J Pharmaceutical Sciences 59 372-376 (1970)
- JF Krowka and BA Jonas, eds, The Personal Care Products Council Microbiology Guidelines, The Personal Care Products Council, Washington, DC (2016)
- DK Brannan, JC Dille and DJ Kaufman, Correlation of in vitro challenge testing with consumer use testing for cosmetic products, Applied and Environmental Microbiology 53 1827-1832 (1987)
- Cosmetic Ingredient Review, Final report on the safety assessment of methylparaben, ethylparaben, propylparaben and butylparaben, J Amer Coll Toxicol 3 147–209 (1984)
- Cosmetic Ingredient Review, Final report on the safety assessment of isobutylparaben and isopropylparaben, J Amer Coll Toxicol 14(5) 364–372 (1995)
- Cosmetic Ingredient Review, Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben and benzylparaben as used in cosmetic products, Intl J Toxicol 27(suppl 4) 1–82 (2008)
- MG Soni, IG Carabin and GA Burdock, Safety assessment of esters of p-hydroxybenzoic acid (parabens), Food Chem Toxicol 43 985-1015 (2005)
- https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_019.pdf (Accessed May 5, 2017)
- https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_074.pdf (Accessed May 5, 2017)
- https://ec.europa.eu/health/archive/ph_risk/committees/04_sccp/docs/sccp_o_138.pdf (Accessed May 5, 2017)
- https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_041.pdf (Accessed May 5, 2017)
- S Oishi, Effects of butylparaben on the male reproductive system in rats, Toxicology and Industrial Health 17 31–39 (2001)
- S Oishi, Effects of butyl paraben on the male reproductive system in mice, Arch Toxicol 76 423–429 (2002)
- S Oishi, Effects of propyl paraben on the male reproductive system, Food Chem Toxicol 40 1807–1813 (2002)
- AM Hoberman et al, Lack of effect of butylparaben and methylparaben on the reproductive system in male rats, Birth Defects Res B Dev Reprod Toxicol J 83 123–133 (2008)
- V Gazin, E Marsden and F Marguerite, Oral propylparaben administration to juvenile male Wistar rats did not induce toxicity in reproductive organs, Toxicol Sci 136 392-401 (2013)
- https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_132.pdf (Accessed May 5, 2017)
- J Boberg, C Taxvig, S Christiansen and U Hass, Possible endocrine disrupting effects of parabens and their metabolites, Repro Toxicol 30 301-312 (2010)
- RJ Witorsch and JA Thomas, Personal care products and endocrine disruption: A critical review of the literature, Crit Rev Toxicol 40(S3) 1–30 (2010)
- R Golden, J Gandy and C Vollmer, A review of the endocrine activity of parabens and implications for potential risks to human health, Critical Rev Toxicol 35 435-458 (2005)
- https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_00d.pdf (Accessed May 5, 2017)
- A Hossaini, JJ Larsen and JC Larsen, Lack of oestrogenic effects of food preservatives (parabens) in uterotrophic assays, Food Chem Toxicol 38 319–323 (2000)
- PD Darbre et al, Concentration of parabens in human breast tumors, J Appl Toxicol 24 5-13 (2004)
- LB Dobbins, S Usenko, RA Brain and BW Brookes, Probabilistic ecological hazard assessment of parabens using Daphnia magna and Pimephales promelas, Environmental Tox and Chem 28 2744-2753 (2009)
- H Yamamoto et al, Preliminary rcological risk assessment of butylparaben and benzylparaben–1. Removal efficiency in wastewater treatment, acute/chronic toxicity for aquatic organisms, and effects on Medaka gene expression, Environmental Sciences 14 73-87 (2007)
- H Yamamoto et al, Aquatic toxicity and ecological risk assessment of seven parabens: Individual and additive approach, Science of the Total Environment 410-411 102-111 (2011)