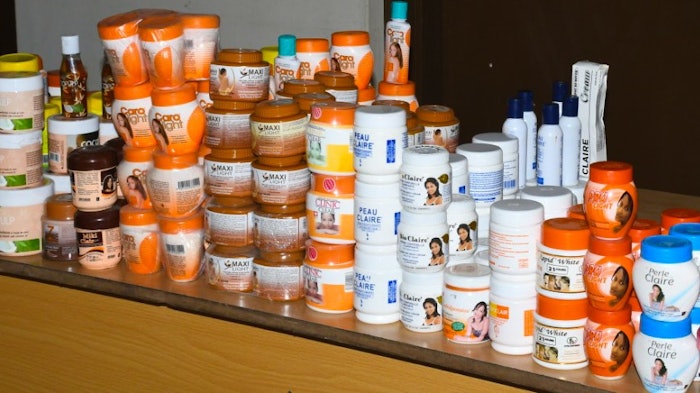
On Oct. 31, 2022, the Kenya Bureau of Standards (KEBS) announced it seized and banned 435 cosmetics from the market. The products include skin brightening lotions, creams, gels and soaps that contain hydroquinone, mercury and its compounds.
This article is only available to registered users.
Log In to View the Full Article
On Oct. 31, 2022, the Kenya Bureau of Standards (KEBS) announced it seized and banned 435 cosmetics from the market. The products include skin brightening lotions, creams, gels and soaps that contain hydroquinone, mercury and its compounds.
See related: FDA Issues Warnings, Pushes Consumer Education of OTC Skin Brightening Products
According to the bureau, the constant use of cosmetic products containing
hydroquinone and mercury plus its compounds can result in rashes, swelling, redness or peeling,
fatigue, weakness, bone pain and skin discoloration. Exposure to high levels of these elements over
long periods of time can lead to serious health problems such as kidney damage, digestive illnesses.
The organization stated, "It is also our responsibility to keep the public informed of the compliance status of products, including
banned cosmetics. . . [W]e have put up
communication on banned products on our website and we do constant surveillance and monitoring in
Kenyan Market ..."
It continued, "Banned cosmetics found will be seized for destruction
at the expense of the owner in addition to any other legal action as provided under the law.
We encourage the public to be vigilant and inform KEBS upon encountering any products suspected to
be substandard."
According to KEBS, mercury, oxidizing agents and hormonal preparations
are used for treating various medical conditions. They are therefore classified as drugs and should be
applied only upon the advice and direction of a medical doctor.
All skin care preparations like creams, lotions, gels, soaps, etc. containing hydroquinone, steroids and
hormonal preparations should be registered by the Pharmacy and Poisons Board of the Ministry of
Health for medical use.
A full list of banned cosmetics is provided in the KEBS announcement. Following are select examples.
Hydroquinone-containing Skin Brightening Lotions
- Jaribu skin lightening lotion
- A3 lemon skin lightening lotion Princess lotion
- Amira skin lightening lotion
- Kiss lotion
- Peau Claire beauty body lotion
- A3 clear touch complexion lotion
- Rico skin lightening lotion
- Clear touch lotion
- Fair white body clearing milk
- Sivoclair lightening body lotion
- . . . etc.
Soaps Containing Mercury and its Compounds
- Movate
- Mekako
- Jaribu
- Tura
- Acura
- Rico
- Fair lady
- Elegance
- Miki
- Jambo
- . . . etc.
Skin Brightening Creams Containing Mercury and its Compounds
- Pimplex medicated cream
- Pimplex medicated cream
- New shirley medicated cream
- Cream preparations containing hydrogen peroxide (H2O2)
- Hot movate gel
- Tenovate
- Soft and beautiful cream
- Unic clear super cream
- Peau claire gel plus
- Peau claire cream
- . . . etc.