The last report on the frequency of preservative use was published two years ago.1 Although at that time approximately 20% more formulations had been registered from previous years, the difference in use of preservatives was not significant.
With the present report, however, some interesting differences have been observed, especially regarding parabens and “nontraditional” preservatives, as will be discussed later.
It is important to note that statistics such as these, which track ingredient usage, are obtained through company submissions. The real reason for companies to submit now is that the US Food and Drug Administration (FDA) recently made the process easier via the Internet; so by simply filling in the forms online and hitting “submit,” companies will find registration and updating to be a snap.
To access current submission forms online, visit the FDA Web site at: www.cfsan.fda.gov/~ocac/ocac2512.html. Here, the FDA refers to the Voluntary Cosmetic Registration Program as the VCRP. The forms available include: 2511–Registration of a Cosmetic Establishment; and 2512–Cosmetic Product Ingredient Statements. Current listings can be updated or formulations can be removed electronically—this becomes an especially important task when products are discontinued. Companies may also request that forms submitted on paper be transferred to an electronic format at this site.
Also, since the last preservative review was published, the Personal Care Products Council (formerly the CTFA) issued its Consumer Commitment Code, which includes mandatory participation in the VCRP. Hence, statistics collected moving forward should be more indicative of current ingredient, formula and product trends.
This year’s report also reports on information submitted to Health Canada. Since cosmetics sold in Canada are required to be registered within 10 days of the first sales, this data will give an accurate view of the preservatives being used. Note that the information included from Canada does not include Drug or Natural Health Product usage for preservatives, which parallels the FDA data excluding OTC drug usage for preservatives.
VCRP Participation
There are some important reasons why every company should participate in the VCRP:
1. One of the best defenses against: regulatory challenges, such as in California; industry-related Congressional hearings such as the Dingell proposal; and continued criticism of the industry by activist groups; is to show that the industry actively participates in the voluntary programs run by the FDA. This shows that self-regulation works.
If registration were to become mandatory, how long would it take for companies to submit and gain FDA approval before finally placing a product on the market? Under this voluntary program, the FDA acknowledges a company’s registration within two weeks. The EU also strongly considered mandatory pre-submissions of product information packages (PIP) or dossiers before companies could place products on the market. Fortunately it was agreed that public access would be allowed to certain parts of the PIP—instead of requiring a pre-approval step.
2. The Cosmetic Ingredient Review (CIR) uses the data collected from the VCRP to determine its priority list. In addition, another even more important use by the CIR is in the case of a material deemed to have “insufficient data” to conclude its safety. Companies that use the ingredient in question may be placed in jeopardy because the FDA, which is actively attending and participating in CIR meetings, has indicated that a cosmetic containing an ingredient with a CIR ruling of “insufficient data” may be required to list the 740.10 label warning. This warning reads: “WARNING: The safety of this product has not been determined.” This requirement is listed in the Code of Federal Regulations (21CFR740.10). Who would buy a cosmetic with this warning on it?
When the CIR faces the problem of insufficient data for an ingredient, the Personal Care Products Council attempts to contact all known users of the material to advise them to submit safety data to the CIR for the ingredient. And how does the council know who uses this ingredient? From these filings. Therefore, another reason to participate, even if a company is not a member of the council, is to gain advanced notification of a potential business threat.
3. The EU requires all ingredients that are used in cosmetics and sold in Europe to be on its inventory list. The original list was an old edition of the CTFA Dictionary, but it quickly became obvious that many ingredients listed in the dictionary were not used. Therefore, the EU began to remove these unused listings from its inventory. How, then, might companies whose ingredients were removed be able to add them to the EU inventory? By proving their ingredients are used.
Evidence of an ingredient’s use can be provided by a government report showing that the ingredient is used; the only such report available to EU officials is the FDA’s report from the VCRP. Hence, any US company that wants to sell its formulations in the EU needs to cover itself by submitting a form to the VCRP for each formulation. In fact, many EU companies that may not necessarily sell products in the United States understand this process and as a result, they register formulations with the FDA.
4. The FDA states that by participating in the VCRP, companies are placed in the pipeline to receive important information if safety issues related to an ingredient arise.
5. OTC drugs are now required to display “Drug Facts” labeling—there is a temporary stay only in the case of sunscreens—and on this labeling, a section marked “Inactive ingredients” should be included. It is within this section that companies are required to list all inactive ingredients in one of two ways.
• If the product makes no cosmetic claims, ingredients are listed in alphabetical order by their drug (USAN) names; or
• If a product makes cosmetic claims, they should be listed in descending order of predominance, down to 1%, using their INCI designations.
One way to prove to a potential inspector that may question a product’s labeling as “cosmetic-drug” is to provide a VCRP receipt from the FDA showing the product is compliant.
Please note that it is important for private label manufacturers to register using Form 2511. Once registered, these manufacturers are added to the listing online, which makes filing by the marketer much easier.
Usage Report for Preservatives: 2007
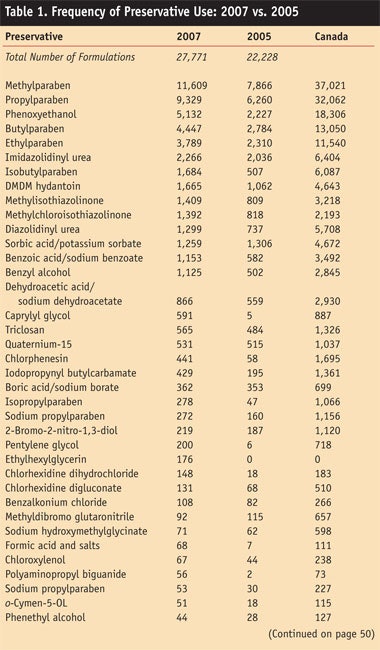
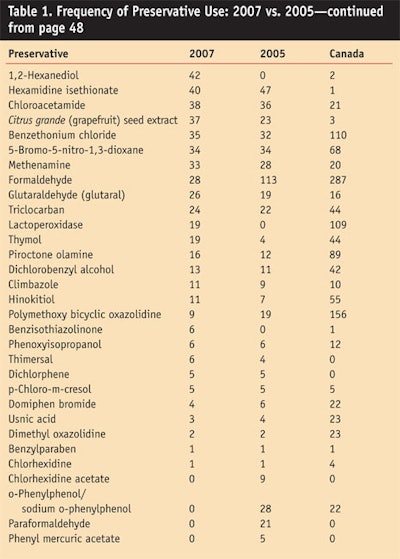
Table 1 and Table 1 cont. shows the current preservatives listing by predominance of use and compares the new data with the last report and current Canadian data. The data from Canada closely follows the voluntary data from the FDA. This correlation substantiates the uses that have been published for several years.
Results
Impressively an approximate 25% increase in overall registration to the VCRP was noted for the present 2007 Frequency of Use report. From this report it is clear that the use of parabens and paraben solutions in phenoxyethanol and imidazolidinyl urea remain the most popular preservatives. Parabens have grown in use, regardless of attacks by media based on questionable science. In fact, a greater than 40% increase in the use of methylparaben was noted.
Some preservatives remain the same, which could indicate inactive registrations that have not been removed, or limited activity. These include paraformaldehyde/formalin, sodium bisulfite, benzethonium chloride and chloroacetamide that was founded as unsafe by the CIR. These listings are likely EU filings.
Others remaining mostly the same are: methenamine; 5-bromo-5-nitro-1,3-dioxane, also likely old filings; o-phenylphenol/sodium o-phenylphenol; grapefruit seed extract (a fraudulent preservative); triclocarban; glutaral; polymethoxy bicyclic oxazolidine; chlorhexidine dihydrochloride; dichlorobenzyl alcohol; phenyl mercuric acetate, which is likely in obsolete formulations that were never deleted; chlorhexidine acetate; domiphen bromide; dichlor
phene; hinokitiol; phenoxyisopropanol; thimersol, which again is probably in obsolete formulations that were never deleted; and benzylparaben, which is probably in obsolete formulations.
Three preservatives that are frequently used in cosmetics for non-preservative purposes may explain their larger number of uses. These include formaldehyde/paraformaldehyde, used in nail hardeners; salicylic acid, used mostly as an exfoliant beta hydroxy acid; and resorcinol that is used as a coupling agent in reactive hair dyes. These materials have been removed from the list along with any preservatives listed in Preservatives for Cosmetics2 since they have zero uses registered in both years.
To properly file a material’s use in formulations, users should be made aware of several important changes. In the case of blends, if methylchloroisothiazolinone and methylisothiazolinone are provided as a mixture, each should be submitted separately. Pure methyl isothiazolinone is now being offered as a commercial material since its approval for leave-on and rinse-off products in Japan and the EU, thus it now is being charted separately, as is methylchloroisothiazolinone.
Another important change to note is regarding combinations of parabens in phenoxyethanol. The most common blend consists of: phenoxyethanol, methyl-paraben, ethylparaben, propylparaben, butylparareben and isobutylparaben. However, many submissions are no longer including the isobutylparaben. These submissions should be updated to include isobutylparaben.
Finally, striking changes have emerged with the growth in popularity of “non-traditional” preservatives introduced in the past years including pentylene glycol; 1, 2 hexanediol; caprylyl glycol and ethylhexylglycerin. These products are sold for purposes other than preservation since they are not on the EU Annex VI list of permitted preservatives or the Japanese list of approved preservatives.
While they are included as moisturizers or for other purposes, these materials do function as traditional preservatives; however, using these ingredients and calling a cosmetic “preservative-free” is false and misleading. It is clear that these ingredients are growing in favor. Expect to see a significant increase in their use the next time this report is published.
Acknowledgement: Special thanks go to the cosmetic branches of the US FDA and Health Canada for their cooperation and for supplying the raw data for this column.
References
1. DC Steinberg, 2005 Preservatives Use: Frequency Report and Registration, Cosm Toil 121 7(65) (Jul 2006)
2. DC Steinberg, Preservatives for Cosmetics, Allured Publishing Corp: Carol Stream, IL USA (2006)