It has been nearly two years since this column reported updates to the EU Cosmetic Directive. During this time, the recast of the directive, known as the 8th Amendment, has been proposed. This amendment currently is in review by the European Parliament and Council of Ministers. If an agreement is reached, the personal care industry will see many sweeping changes, as reported in the June 2008 edition of this column.
One of the justifications for the total revision of this directive is the belief that it has been changed 55 times due to inconsistencies. This is misleading because although the fundamental regulations have been amended seven times, the majority of changes were Adaptations to Technical Progress (ATP). These changes reflect the advancement of knowledge-whether they are new developments in UV filters or preservatives, or restrictions on ingredients. Also included in ATPs are additions to the list of not permitted ingredients. Thus, stating that a revision of the directive is necessary due to inconsistencies is like saying the industry should return to the Dark Ages and freeze all learning and discovery.
Previous ATPs were referenced numerically but more recently, a date-based system was implemented. The latest eight ATPs are reviewed below.
Recent ATPs
Fluoride in oral care: In Annex III of the Cosmetic Directive, the List of Substances Which Cosmetic Products Must Not Contain Except Subject to Restrictions and Conditions Laid Down (the restricted ingredient list) includes the fluoride-containing compounds allowed in oral care products (items 26-43). On Aug. 29, 2007, an ATP was introduced that changed the labeling for products containing these compounds to require the following phrases: “Children of 6 years and younger: Use a pea-sized amount for supervised brushing to minimize swallowing. In case of intake of fluoride from other sources, consult a dentist or doctor.” The one exception for this requirement was in the case where the label already stated “for adult use only.”
Ingredients in permanent hair dyes: This ATP went into effect on Aug. 29, 2007, and added 85 chemicals (item numbers 1244-1328 on the list) to the Annex II List of Substances Which Must Not Form Part of the Composition of Cosmetic Products (the banned list). This ATP also changed Annex III, References 8 and 9, to reflect the ingredient additions to Annex II. These changes all concern ingredients used in permanent hair dyes. Since they are primarily listed by chemical name in the ATP, a cross-reference of some of their INCI designations is shown in Table 1. This table includes the number of formulations in the United States that currently use these ingredients.
Annex III, Reference 8, was amended to read: “and under reference numbers 1309, 1311 and 1312 in Annex II,” after phenylenediamine, its N-substituted derivatives and its salts; and N-substituted derivatives of o-phenylenediamine, with the exception of those derivatives listed elsewhere in Annex II. Reference 9 added prohibited ingredients 1310 and 1313 to Annex II.
Hair dye substances: This ATP, dated Nov. 22, 2007, extended the renewal deadline for the 42 provisional hair dye substances listed in Annex III, part B, until Dec. 31, 2009.
Glyoxal in finished products: On Feb. 15, 2008, this ATP added glyoxal as number 102 to Annex III, with a concentration limit of 100 mg/kg in finished products. This ATP allows the continued use of glyoxal since it is listed as a Category 3 CMR and must therefore be reviewed and permitted by the SCCP. Glyoxal is listed as a permitted preservative under Annex VI.
Fragrance ingredients and benzyl alcohol: This ATP added a significant number of fragrance ingredients to Annex III on Apr. 3, 2009, including their limitations and other requirements. Table 2 highlights a few prominent additions, here. The first change in this ATP was to correct Annex II item 1136, which currently reads: “Peru balsam (INCI: Myroxylon pereirae); CAS 8007-00-9,” when used as a fragrance ingredient. This was replaced with: “Exudation of Myroxylon pereirae (Royle) Klotzch (Peru balsam, crude); CAS 8007-00-9,”when used as a fragrance ingredient.
The second change was to delete item 68 in Annex III, which lists benzyl alcohol as a fragrance allergen. Benzyl alcohol is already on Annex III as a solvent in item 45, so the two were combined. In addition, changes were made to three other fragrance allergens, including hydroxycitronellal, isoeugenol and d-limonene (see Table 3).
Hair dye ingredients: On Sept. 23, 2008, another ATP added 41 hair dye ingredients to Annex II from Annex III. These substances were all delisted because no supporting safety data was available to support their use in hair dyes. Included were colors remaining in the Annex IV List of Coloring Agents Allowed for Use in Cosmetic Products. This list is shown in Table 4.The changes and additions to Annex II resulted in changes to Annex III, and included: the deletion of diaminophenols and hydroquinone as a hair care ingredient; and the delisting of the provisionally allowed status for Basic Blue 26 (CI 44045), FD&C Red No. 4 (CI 14700) as a colorant, and Basic Violet 14 (CI 42510) as a hair dye.
UV filters: This ATP went into effect on Dec. 12, 2008, and deals with sunscreens in the Annex VII List of UV Filters Which Cosmetic Products May Contain. It delists PABA, known as 4-aminobenzoic acid or the drug name aminobenzoic acid, which was permitted up to 5%. This required Annex II entry 167 to be changed from “Esters of 4-aminobenzoic acid with the free amino group, with the exception of that given on Annex VII,” to “4-aminobenzoic acid and its esters, with the free amino group.” This second and minor change was to eliminate a restriction of the UV filter diethylamino hydroxybenzoyl hexyl benzoate for use only as a sunscreen.
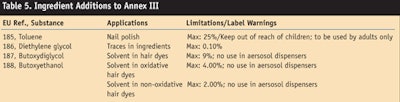
Toluene and hair dye ingredients: This ATP, dated Feb. 4, 2009, prohibited two ingredients and added four ingredients to Annex III. New inclusions on the prohibited list are EU ref. 1370, diethylene glycol; and ref. 1371 phytonadione, also known as vitamin K1. The four ingredients added to Annex III are shown in Table 5.
Comments: Regulation in the United States
In the United States, cosmetic regulations are simple—manufacturers are required to place safe products on the market. If they do not, the US Food and Drug Administration (FDA) can take action against them but usually the lawyers get there first. It is baffling how consumers will not buy cosmetics containing traces of allegedly “unsafe” ingredients (based on questionable science), yet they certainly buy unsafe foods that cause disease and even death-and nongovernmental organizations (NGOs) are not protesting this. However, when it comes to cosmetics, NGOs want either all cosmetics and ingredients pre-approved by the government before they are placed on the market or they want no cosmetics at all. The worst sin committed by the personal care industry is the false, exaggerated (“puffery”) or misleading promotion of products.
However, the industry has done severe damage to itself by basing advertisements for products on claims of what the products do not contain rather than the benefits they provide. This began in the early 1980s with “PABA-free” claims, and the industry will see the consequences of advertising in this manner. For example, a bill was introduced in the New York State Assembly on Mar. 13, 2009, that if passed will require all cosmetics sold in New York state to include the warning: “Caution—Use only as directed. Exposure to an ingredient contained in this product has been linked to health problems, including certain cancers, reproductive problems and hormone disruption. Keep out of the reach of children.”
Manufacturers would be required to apply this label to products containing any amount of cyclotetrasiloxane, cyclopentasiloxane, phthalates, triclosan, parabens or synthetic chemical musks. The penalty is a fine of up to US $500, which is likely per item of commerce. The premise of this bill is based on unfounded science because if exposure to these materials at the levels used in cosmetics did in fact risk public health, the FDA would have taken action.
An EU/US Comparison
The EU does not face the same cosmetic regulation issues as the United States. This is because the government believes it necessary to require cosmetic companies to put only safe products on the market, to avoid unsafe products reaching the market, and that it is necessary to dictate to cosmetic chemists which ingredients not to use.
NGOs in the United States regularly cite the fact that the EU has now prohibited 1,371 ingredients, compared with the 10 ingredients banned in the United States, although a few others are restricted. The FDA only preapproves colors, while the EU preapproves colors, preservatives and a whole list of restricted ingredients. Of the 1,371 ingredients prohibited in Annex II, the only ones that have ever been or are currently formulated into cosmetics are: brucine, found in SD alcohol-40 in the United States; choline chloride; cholecalciferol (vitamin D); secondary alkanolamines and their salts such as DEA cetyl phosphate; cells, tissues or products of human origin; dibutyl phthalate, used only in nail polish and now mostly unused due to NGO pressure-even the EU’s Scientific Committee claimed it was safe for this use; petrolatum, except when it is shown to be produced from a noncarcinogenic origin; and bishydroxyethyl biscetyl malonamide.
There have been no injuries reported in the United States as a result of these ingredients. As for the other 1,361 ingredients, no one even thinks of using such chemicals as cyanides, chlorine gas, diesel fuel, radioactive chemicals, and so on, and a rule prohibiting these ingredients is not needed.
In regard to ingredient safety, as the author has explained to the FDA, formulators can take chemicals that are unsafe under certain conditions and use them safely in cosmetics because chemicals are not inherently safe or unsafe-it is the final formulations that must be safe. As an example, the author described water. Approximately 5 g of water in the lungs will result in death (i.e., drowning) but the industry certainly has no problems with water in cosmetics. Water is also necessary to sustain life.
Discussion
In the described ATPs, the EU has banned a number of hair dye ingredients in formulations that the industry does not use, and for which it does not submit safety data. Therefore, the EU has prohibited what is not being used based on nonexistant safety data.
The EU also has added a significant number of fragrance ingredients to its restricted list that reflect the standards of the International Fragrance Association (IFRA). Adding all these fragrance components to the restricted list and establishing limits on their use and trace contaminants really does not add to the safety of cosmetics. It does, however, add clutter to Annex III and another document that fragrance suppliers must provide-a statement attesting to their conformance with IFRA and now Annex III standards.
The addition of PABA to the prohibited list is another example of an ingredient that is not used. At one time it was the only UV filter allowed in the EU, Japan, the United States, Canada and Australia, but the relentless “PABA-free” drive, married to the fact that it is not a good UV filter, has succeeded in eliminating its use in the EU. NGOs will likely campaign at the FDA to ban PABA from US sunscreens as well.
The banning of diethylene glycol is in response to adulterated toothpaste exported from China. When ingested, it is toxic but most cosmetics are not ingested, so why not just prohibit it from oral care products? Only seven formulations currently registered with the FDA contain it, so its loss would have minimal impact. Additionally, it was used safely as a solvent for some fragrances.
Vitamin K1 is a different story. Here, severe allergic reactions and even death resulted from exposure to parts-per-millions of this material in creams.1 Since its primary cosmetic function was “it sounds good on the label,” this is a case where the risks justify its removal from cosmetics. It’s interesting to note there have not been any calls from the NGOs to ban this ingredient. The Environmental Working Group’s Web site, www.cosmeticsdatabase.com, gives it a rating of 2—their lowest hazard ranking, where 0 is best and 10 is worst.
Keeping everyone up to date on EU changes is important but these eight changes really accomplished little. At press, the Parliament and Council announced an agreement on the new 8th Amendment. These changes are major and will go into effect in 2012-and doubtless, will keep this author very, very busy.
References
Send e-mail to [email protected].
1. SCCP Opinion, June 24, 2008, http://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_140.pdf (Accessed Apr 3, 2009)