As is generally known, all sunscreens—chemical or mineral, nano or non-nano—are regulated by the U.S. Food and Drug Administration (FDA) and other national regulatory bodies, and have been found to be safe. Chemical sunscreens have been used by the public for more than 65 years,1 and mineral sunscreens for more than 20 years.2 However, there is a growing public hysteria about the possible risks nano-sized particles in sunscreens pose.
This fear has been promoted mainly by NGOs such as Friends of the Earth (FOE) and its allies, even in the face of overwhelming evidence from independent panels of experts that nanoparticulate titanium dioxide and zinc oxide are safe sunscreen actives. Nanoparticles and nanomaterials are ubiquitous. They are present in the atmosphere, and have presumably been there for hundreds of millions of years. Animals, including humans, have evolved in their presence.
To gain some perspective, consider the extent to which individuals are exposed to nanoparticles every day. Inhalation is, by far, the most significant route by which nanoparticles reach internal organs. According to a report by the Royal Society and Royal Academy of Engineering,3 there are estimated to be between 106 and 108 nanoparticles per liter of air, and the average person inhales between 500,000 and 50 million nanoparticles with each breath—i.e., about 6 million to 300 million nanoparticles per minute.
Also, nanoparticles are major components of clays and soils. Milk contains nanoparticles, and ricotta cheese consists of suspensions of nanoparticles.4 These are present in the body as protein molecules and enzymes; indeed, humans cannot live without them.
Nanoparticles of carbon were first manufactured nearly 100 years ago,5 The fact that sunscreen ingredients are nano-sized should not frighten anyone—after all, it could be said that humans are made up of a collection of nanoparticles known as proteins. Interestingly, the FOE campaign focuses exclusively on sunscreens and cosmetics6 using zinc oxide and titanium dioxide nanoparticles while ignoring sunscreens containing molecular UV absorbers, which themselves have dimensions that qualify as nanoparticles. In fact, it is arguable that all active ingredients used to absorb UV radiation in sunscreens are actually nanomaterials and should be classified as such.
This paper reviews important issues regarding classifications for UV actives. It also emphasizes the need for a uniform classification and labeling system to cover all nano ingredients. The definition of nanoparticles is not questioned here; however, the selective manner by which regulatory agencies apply the definition to different materials is.
Defining Nanoparticles and Nanomaterials
As noted, nanoparticles themselves are not new; however, the terms nanoparticle and nanomaterials coined to describe them are new, as are the abilities to control the synthesis of, measure and design these very small particles and their properties. There is actually no common definition of nanoparticles or nanomaterials, and it is unlikely there will ever be.5 The International Organization for Standardization (ISO) provides an inclusive definition of nanoparticles/nanomaterials as materials having any external dimension, internal structure or surface structure in the nanoscale, defined as a size range between approximately 1 nm to 100 nm.7 Some organizations have adopted a size of less than 1 nm as the lower limit. For example, the European Medicines Agency8 and the U.K.’s Royal Society and Royal Academy of Engineering9 define the size of nanoparticles as being from 100 nm down to the atomic level (~0.2 nm). This definition includes molecules, but not atoms or ions, as nanoparticles.
Some organizations include additional criteria in their definition of nanomaterials,5, 9 which tend to be application-specific. Of relevance to this paper, the European Cosmetics Product Regulation10 defines nanomaterials as “insoluble or biopersistent and intentionally manufactured materials with one or more external dimensions, or an internal structure, on the scale of 1 nm to 100 nm.” Thus, to qualify for the nano rating in Europe, the nanomaterial must be insoluble or biopersistent, regardless of the safety of the product. Whether or not additional criteria are added to their definition, listings of nanomaterials are overwhelmingly inorganic, with little or no mention of organic nanomaterials apart from carbon nanoparticles.
There is ambiguity over the meaning of terms such as biopersistence and solubility used in defining nanoparticles.11 In the language of organic chemistry, to dissolve molecules actually means to disperse them. Here, molecules are not chemically altered by dissolution. For example, to formulate with molecular UV actives, a critical step is to “dissolve” the actives into the formulation. Special “solubilizers” are used to ensure these actives are fully “dissolved.” However, in the language of inorganic chemistry, to dissolve an inorganic compound such as zinc oxide means it will be chemically changed into Zn+2 and OH- ions. The EU Scientific Committee on Consumer Safety guidance for the safety assessment of nanoparticles in sunscreens12 suggests that solubility means disintegration of a nanomaterial in an aqueous medium or biological environment into molecular components with the loss of nano features.
In this paper, it is argued that whether a material is molecular or supramolecular, organic or inorganic, biopersistent or not biopersistent is irrelevant as to whether or not it is a nanoparticle: The word nano(meter) refers only to size. If other criteria are involved, then it should be made very clear in product specification and labeling, for example, “biopersistent nanoparticles.”
Molecular UV Actives are Nanoparticles
Now consider molecular UV actives. The word molecule originates from the French word molécule, meaning “extremely minute particle,” which was first used in 1704.13 Thus, a molecule with any dimension less than 100 nm is a nanoparticle. This of course is a literal definition. In this paper, molecules and polymers of sizes 1–100 nm are considered molecular or polymeric nanoparticles.
The size of a molecular UV active can be determined by its molecular weight. Erickson14 showed that the volume of a protein molecule, Vm, can be calculated from its molecular weight and density using the equation:
Vm (nm3) = 0.00166 M/ρ Eq. 1
where M is the molecular weight in Daltons and ρ is the density of the molecule (g/cm3). The numerical factor in Eq. 1 converts Daltons to grams (Da = 1.66 x 10-24 g) and cm3 to nm3. While this formula was developed for proteins, it is equally applicable to UV actives. To calculate the linear dimension of the molecule, knowledge of the shape—i.e., equiaxed, planar or linear—of the molecule is also required.
Figure 1 shows the atom arrangement15 of a common molecular UV active, avobenzone, which is used in many sunscreens as a UVA absorber. The molecular weight of avobenzone is 310.4 Da and its density is 1.03 g/cm3. Using Eq. 1, Vm (nm3) = 0.00166 x 310.4/1.079 = 0.566 nm3. Assuming it is a spherical shape gives the diameter of the molecule equal to 1.02 nm. From this, it is clear that the avobenzone molecule fits the nanoparticle definition.
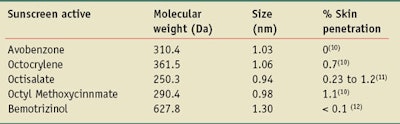
Table 1 gives the sizes of selected currently approved molecular UV actives calculated using Eq. 1, assuming the molecules have a spherical shape. In most cases, however, such as for the avobenzone nanoparticle, the molecules have an elongated shape. This makes the length of the molecules greater and the minimum dimension of the molecules smaller than the values given in Table 1.
The single molecule sizes of the UV actives listed in Table 1 are all less than 2 nm, and of the 27 molecular actives approved by the FDA and Australian and European regulators, all are less than 100 nm in size. Therefore, they all should be classified as nanoparticles. Further, not only do these molecular UV actives look like nanoparticles, they also exhibit particle-like behavior—e.g., sedimentation, the ability to be filtered, agglomeration and dispersibility. These are all particle-like characteristics exhibited by molecules the size of UV actives.14
Contrary to molecular actives, the particle sizes of inorganic particles such as zinc oxide and titanium dioxide are not determined by molecular weight. Rather, their particle sizes can be varied to achieve the desired properties. With zinc oxide, particle sizes greater than 30 nm are needed to achieve a broad-spectrum rating for sunscreen.16 Compared with the molecular weights shown in Table 1, a 30-nm diameter zinc oxide nanoparticle corresponds to 48,000,000 Da.
For both molecular and inorganic actives, aggregated particles should be classified as nanomaterials under the current classification, regardless of their overall size, due to the dimensions of the primary particles/molecules from which they are built. Further, vesicles such as liposomes or polymer nanocapsules are also examples of nanomaterials used in cosmetics.
Dermal Penetration
The main safety concern over the use of zinc oxide and titanium dioxide nanoparticles, raised repeatedly by FOE, is the potential for penetration through the skin and entry into the body’s systems. Simply put, the skin can be considered a porous membrane through which particles can flow if they are smaller than the size of the pores. In fact, due to their very small size, molecular nanoparticles currently used in sunscreens exhibit dermal penetration.2, 17-20 So how small is sufficiently small for a particle or nanomolecule to penetrate the skin?
Dutch scientists Bos and Meinardi21 argued that, based on the size of molecules used for dermal drug delivery, if the molecular weight of the molecule is less than 500 Da, it can penetrate the skin. They based their conclusion on three facts: virtually all common contact allergens are under 500 Da; most of the common pharmacological agents applied in topical dermatotherapy are smaller than 500 Da; and all known topical drugs used in transdermal drug-delivery systems are under 500 Da.
A survey of the 27 approved UV actives previously described shows that 20 of the 27 compounds, i.e., 74%, have molecular weights less than 500 Da, thus passing the 500-Da rule; 13 of these are less that 300 Da. Using Eq. 1, 500 Da corresponds to a ~1.6-nm diameter spherical particle.
It has long been known that molecular nanoparticles used as UV actives in sunscreens penetrate the human skin. This does not mean they are dangerous to human health. In fact, as far back as the 1978 FDA sunscreen monograph,2 the percutaneous absorption of UV actives and the consequences of skin penetration were considered in some detail. The monograph even recognized that the sizes of the UV actives used were sufficiently small to penetrate the skin, stating that “molecules less than 1,000 Da in size are usually well-absorbed in the skin.” A considerable portion of the 1978 monograph was devoted to the results of safety tests on the UV actives being evaluated for approval, and this focus on product safety has continued to the present day.22, 23
In comparison to molecular UV actives, there is no substantiated evidence that zinc oxide or titanium dioxide nanoparticles penetrate the human skin.16, 20, 24 Nanoparticles of zinc oxide and titanium dioxide are more than 10 times larger than molecular UV actives, and simply too big to penetrate human skin. Using a golfing analogy, it would be equivalent to trying to knock a football into a golf hole.
The Hazards of New Terminology
Given the much smaller size of molecular UV actives, compared with zinc oxide or titanium dioxide nanoparticles, and the well-documented dermal penetration of many of the molecular actives, it seems remarkable that the FOE focuses so much attention attempting to discredit the use of inorganic nanoparticles in sunscreens. Equally, one might also ask why UV actives have not been classified as nanoparticles by the regulatory authorities. The answer to the latter question is very simple: the development of sunscreen actives predated the era of nanotechnology by many decades.
Nanoparticle was not a word in the scientific lexicon of the day when the first sunscreens were developed and regulations promulgated. Interestingly, the publication of the 1978 sunscreen monograph2 coincided with the year that the first U.S. patent was granted containing the word nanoparticle. Had the emergence of nanotechnology occurred 40 years earlier or sunscreens developed 40 years later, there would be no controversy today; all UV actives would be classified as nano by virtue of their size and particle-like behavior. This is clearly the case in the closely related field of drug delivery, where new drug molecules of similar size and shape as UV actives are routinely described and classified as nanopolymers and nanomolecules. Given the hysteria over the nano word, it is perhaps understandable why there is no rush by sunscreen manufacturers or regulatory bodies to change the status quo.
Nano Labeling
The labeling of products that contain nanoparticles is currently a controversial topic. Some groups want all products containing nanomaterials to be labeled as such, and for sunscreens and cosmetics in particular, there have been numerous calls for nano labeling.
Currently there appears to be no consensus on labeling by regulatory authorities. In Europe and New Zealand, regulations have been adopted requiring cosmetic manufacturers to label products containing nanoparticles,25, 26 although the labeling simply states nano is only required for biopersistent or insoluble nanoparticles. This of course raises questions discussed earlier in this paper regarding the ambiguity and applicability of biopersistence and solubility as applied to UV actives.
In the United States and Australia, regulatory authorities do not require nano labeling. In fact, in Australia, the Therapeutic Goods Administration has gone a step further by forcing sunscreen manufacturers to stop advertising product as “not nano”27 since, evidently, advertising as “not nano” could cause “fear or distress” to consumers, therefore breaching the Australian advertising code.
Nano labeling sounds like a good idea in principle, but uniform recognition of all nanoparticles would mean that all sunscreen products should be labeled as containing nanoparticles. What, then, would be the purpose? To scare the public into not using sunscreen? Given the public focus on dermal penetration, to not require labeling of nanoparticles that are well-known to penetrate the skin but are not biopersistent while at the same time requiring labeling of inorganic nanoparticles, which on the basis of all evidence to date do not penetrate the skin, has no logic.
Summary
This paper has argued that molecular UV actives used in sunscreens should be classified as nanoparticles on the basis of their size and particle-like behavior. If regulatory bodies are to adopt a size-based classification of UV actives, it must be done retrospectively and uniformly across all actives. Otherwise, the kind of “nanoscare” activities28 currently employed by NGOs will continue. The major problem is that each one of these sensational attacks undermines the confidence of the public in using sunscreens. It is of critical importance that public confidence in the safety of sunscreens not be destroyed by over-the-top claims that are at variance with all credible scientific evidence. This author is not aware of any deaths attributable to the use of sunscreen. On the other hand, there are countless deaths associated with failure to use sunscreen or cover up while in the sun.
Acknowledgements: The author would like to thank Malcolm Nearn, PhD, for many valuable discussions and comments during the course of this work.
References
1. www.pizbuin.com/en/our-heritage (Accessed Sep 9, 2013)
2. Sunscreen products for over-the-counter human use, 21 CFR Part 352, Federal Register 43 166 (Aug 25, 1978) pp 38206-38225
3. Nanoscience and Nanotechnologies: Opportunities and Uncertainties, Royal Society and the Royal Academy of Engineering, Latimer Trend Ltd., Plymouth, UK (Jul 2004)
4. Nanotechnology, The Cosmetic, Toiletry and Perfumery Association Ltd., www.thefactsabout.co.uk/content.asp?pageid=1&menuname=Nanotechnology&menu=hidden (Accessed Sep 9, 2013)
5. G Lövestam, H Rauscher, G Reobben, BS Klüttgen, N Gibson, J-P Putaud and H Stamm, Considerations on a definition of nanomaterial for regulatory purposes, European Commisson, EUR 24403 EN (2010)
6. www.foe.org/projects/food-and-technology/nanotechnology (Accessed Sep 9, 2013)
7. Nanotechnologies–Vocabulary part 1, core terms, ISO TS 8004-1, www.nano.gov/you/standards (Accessed Sep 9, 2013)
8. Reflection paper on nanotechnology-based medicinal products for human use, EMEA/CHMP/79769/2006, European Medicines Agency, www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2010/01/WC500069728.pdf (Accessed Sep 9. 2013)
9. Nanoscience and Nanotechnologies, The Royal Society and the Royal Academy of Engineering, www.nanotec.org.uk/report/chapter2.pdf (Accessed Sep 9, 2013)
10. EU Regulation (EC) No. 1223/2009 on cosmetic products, European Parliament and Council, https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:342:0059:0209:en:PDF(Accessed Sep 9, 2013)
11. The European Commission, Scientific Committee on Emerging and Newly Identified Health Risks, Scientific basis for the definition of the term “nanomaterial,” ec.europa.eu/health/scientific_committees/emerging/.../scenihr_o_030.pdf (Accessed Sep 9, 2013)
12. Scientific Committee on Consumer Safety, SCCS guidance on the safety assessment of nanomaterials in cosmetics, https://health.ec.europa.eu/scientific-committees_en (Accessed Sep 9, 2013)
13. Collins English Dictionary—Complete and Unabridged, 10th edn 2009, William Collins Sons and Co. Ltd., now HarperCollins, NY (1979)
14. HP Erickson, Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration and electron microscopy, in Shulin Li, ed, Biological Procedures Online 11, 32-48 (2009)
15. Avobenzone 3D, https://commons.wikimedia.org/wiki/File:Avobenzone-3D-spacefill.png(Accessed Sept 9, 2013)
16. Scientific Committee on Consumer Safety Opinion on ZnO (nano form) (Sep 18, 2012), https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_103.pdf(Accessed Sep 9, 2013)
17. CGJ Hayden, SE Cross, C Anderson, NA Saunders and MS Roberts, Sunscreen penetration of human skin and related keratinocyte toxicity after topical application, Skin Pharmacol Physiol 18 170-174 (2005)
18. KA Walters et al, Percutaneous penetration of octyl salicylate from representative sunscreen formulations through human skin in vitro, Food Chem Toxicol 35(12) 1219-25 (1997)
19. A Mavon et al, In vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreen, Skin Pharmacol Physiol 20(1) 10-20 (2007)
20. GJ Nohynek, J Lademann, C Ribaud, and MS Roberts, Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety, Crit Rev Toxicol 3 251–277 (2007)
21. JD Bos and MM Meinardi, The 500 Dalton rule for the skin penetration of chemical compounds and drugs, Exp Dermatol 9 165-169 (2000)
22. Safety of nanomaterials in cosmetic products, Guidance for industry, US Department of Health and Human Services (Apr 2012), www.fda.gov/Cosmetics/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/ucm300886.htm (Accessed Aug 15, 2013)
23. FDA Regulation of Nanotechnology Products, www.nanopharmaceuticals.org/FDA.html (Accessed Aug 15, 2013)
24. Literature review on the safety of titanium dioxide or zinc oxide nanoparticulate in sunscreens, Department of Health and Aging, Therapeutic Goods Administration, Government of Australia, www.tga.gov.au/industry/sunscreens-nanoparticles-review-2013.htm (Accessed Sep 9, 2013)
25. Regulation (EC) No. 1223/2009 of the European Parliament and Council on cosmetic products, https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:342:0059:0209:en:PDF(Accessed Aug 15, 2013)
26. Environmental Protection Agency, www.epa.govt.nz/news/erma-media-releases/Pages/Cosmetic-Products-regulations-updated.aspx (Accessed Aug 15, 2013)
27. TGA Complaints Resolution Panel Determination, 2010-07-006 (Apr 11, 2010), www.tgacrp.com.au (Accessed Aug 15, 2013)
28. DM Berube, Rhetorical gamesmanship in the nano debates over sunscreens and nanoparticles, J Nanopart Res 10 23–37 (2008)