The word hyaluronic is derived from the Greek hyalos meaning “glass” or “transparent” and refers to the vitreous humor, the ocular tissue from which it was first isolated by Karl Meyer and colleagues in 1934. It was later located in many other animal tissues, i.e. synovial fluid, cartilage and the umbilical cord, where it has the same structure and biological activities, described in this article. Hyaluronic acid (HA)is a linear polysaccharide of high molecular weight that belongs to the family of mucopolysaccharides or glycosaminoglycans (GAGs), the physiological constituents of the dermal connective tissue in the extracellular matrix. In adult humans, the total amount of HA is equal to approximately 15 g, half of which is found in the skin.
At the skin compatible pH of 7, the carboxylic groups of HA are almost totally ionized, thus forming a polyanionic structure that imparts excellent hydro-coordinating properties in skin. This structure is mainly produced by skin keratinocytes and fibroblasts whose complete renewal cycle takes 2–4.5 days1—a much shorter time than the collagen cycle, which takes 60–70 days.2
Due to the functions HA serves in skin homeostasis, both its synthesis and degradation must be rapid and wellcontrolled by biological mechanisms. Indeed, it is an active physiological polymer that not only fulfills structure and filling functions, but also supplies water in the skin. In fact, due to its chemical structure, it is able to retain large amounts of water; for example, one gram of HA may act as a ligand for up to 6 L of water.3 Further, HA forms a scleroproteic sheath on fibrous protein, thus ensuring appropriate lubrication.
Similar to a biological sponge, HA resists compression and imparts structure to the dermis. This is due to its electrostatic interactions with collagen fibers, matrix proteins and other GAGs that form large-sized, “bottlebrushshaped” aggregates called proteoglycans. The structural organization of the extracellular matrix stabilizes the orientation of collagen fibers, preventing them from becoming too close and forming links among fibrils, in turn developing into insoluble collagen and toughening the skin, as occurs with age.
Finally, HA, fully surrounded by its hydration sphere, facilitates the extracellular transport of ions, solutes and metabolites, much like a chromatographic column. In addition, it affects the migration, differentiation, growth and adhesion of cells; angiogenesis; and control of the immune response.1, 3–6
Applications
HA has been used in the medical field for its biological functions and safety; its use is in ophthalmic surgery and orthopedics specifically is well-documented. In plastic surgery, preparations of reticulated HA are injected to fill wrinkles, scars and irregular skin surfaces, as well as to shape the lips and the face. Its application in cosmetics began more than 500 years ago and increased with the discovery that HA levels in skin decrease with age, resulting in the formation of wrinkles, a decrease in elasticity, and the thinning and drying out of skin.7–10
While little information exists correlating skin photo-aging with the metabolism of HA,1, 11 UV rays are known to act directly on cutaneous HA, depolymerizing it by means of reactive oxygen species (ROS) generated in situ; however, large amounts of HA may also act as scavengers of ROS.
Throughout the course of its history, HA has moved into the circle of functional substances for skin care. Produced synthetically today, the raw material is estimated to be worth more than US$ 1 million/kg.12 Its chemical and physical characteristics, natural and safe origin, wide range of biological functions, and ease of use have ensured its success; indeed, it is easily dispersed in water and forms a viscoelastic and highly lubricating gel.
Applied topically, HA forms a moisturizing film that is permeable to both air and light, is non-sticky, and that imparts a variable tensing effect on skin dependent upon its concentration and molecular weight. Due to its capability to retain and slowly release water, HA can improve skin elasticity and plasticity and barrier function as well as reduce transepidermal water loss. In addition, it is surmised that besides acting on the skin’s surface, HA serves as a messenger to the deeper skin layers.7
Finally, the cicatrizing activity of HA is believed to be correlated with its influence on cell migration and chemotaxis, and its topical use on skin ulcers has proven benefits over traditional treatments including hyperbaric oxygen and electrical stimulation. Moreover, HA-based creams have been shown to prevent stretch marks or striae distensae that occur during pregnancy, making them less visible and reducing scarring.7
Recent Developments
As noted, the properties of HA vary according to its molecular weight. For instance, it has been found to form a nonocclusive film at values of approximately > 5 x 105 Da. At lower molecular weights (LMW),13 it imparts cell renewal benefits by attracting inflammatory cells, in turn stimulating the production of cytokines and growth of endothelial cells. LMW HA also facilitates cell proliferation, thus affecting tissue repair.14
Further, new findings show that the topical application of HA induces an antimicrobial response, suggesting its use as a physiological stimulant for inherent antibacterial activity in the skin.15 Due to its functional groups, HA can be used to develop reticulated hydrogels that carry and release DNA and active pharmaceutical principles. In addition, it can be directly linked or bonded to active substances or employed in preparations utilizing microcapsules as plasmide carriers.11
Finally, the bond between HA and butyric residues is of particular interest for creating molecules with cellprotective activities. This unique system is endowed with a double structure that can simultaneously combine the advantages of two active components;16 this system is explored here.
Sodium Butyrate
Short-chain aliphatic carboxylic acids can influence various biological effects, including cellular response to specific hormones and changes in gene expression. Although the effect of sodium butyrate on the synthesis rate of HA in fibroblasts has not been established, it appears to have a dosedependent regulating effect; at low doses (1 mM), it induces significant stimulation (up to 27%) while at higher concentrations (2–10 mM), it has an inhibitory effect.17, 18
The effect of sodium butyrate to induce apoptosis in keratinocytes also has been studied.19 Moreover, research on fibroblast cultures has shown sodium butyrate to induce the biosynthesis of collagen through different metabolic processes. First, it improves the expression of IGF-IR, an important receptor for collagen formation, and reduces the activity of the inhibiting enzymes protein kinase MEK 1/2 and MAPK. Furthermore, it stimulates prolidasis, an enzyme present in the final stage of protein catabolism, which is essential for re-using the amino acid proline in collagen neo-synthesis and cellular growth.20, 21
Thanks to antiproliferative and cell differentiation activities, sodium butyrate has been recommended for the prevention and treatment of preneoplastic lesions; however, its use as a potential cell growth inhibitor in the clinical field is challenging due to the concentrations required in vivo. Indeed, its half-life is short (approx. 5 min) due to rapid metabolism and excretion (see Figure 1). Thus, in order to increase its presence in biological tissues, new derivatives of sodium butyrate have been developed, such as monosaccharide, triglyceride, phenolic derivatives and alkoxyalkyl derivatives of carboxylic acids. Consequently, esterification with HA was of high clinical interest.22
Synthesis of HA Butyric Esters
To synthesize HA butyric esters, sodium hyaluronate molecules (approx. molecular weight 400 kDa) are solubilized in organic solvent and treated with butyric anhydride in the presence of organic alkalis at room temperature (RT) for 2 hr. This solution is neutralized with sodium bicarbonate. The degree of substitution with butyrate groups may range in weight from 1.8% to 28.4%, depending on the amount of butyric anhydride employed.22 This process is depicted in Figure 2.
HA Butyric Esters
The main advantages of employing butyrate residues with HA include high biocompatibility and binding capability of the conjugated HA with the membrane receptor CD44. This receptor is expressed by standard epithelial and mesenchymal cells to serve an important role in immune recognition, the migration of lymphocytes, and cell-to-cell and cell-to-matrix interactions;23 it is overexpressed in tumor cells. Thus, from a clinical standpoint, the biological effects of butyric esters are assessed in terms of growth inhibition of tumor cells.23–25
Butyrate ester bonds also complement the inherent efficacy of HA by optimizing its biological features. For instance, esters of HA mixed with butyric or retinoic acids have been found to induce the differentiation of pluripotent embryonic stem cells into heart cells.26, 27 Further, butyrate ester bonds affect the degradation time of native HA; the turnover of HA in the epidermis is long,28 its degradation in the dermis is faster, and its turnover is very fast, i.e. 3–5 min, in the liver and bloodstream.28, 29
However, when submitted to in vitro degradation tests at concentrations of hyaluronidase similar to those found in the skin, butyric esters exhibited higher enzymatic resistance than HA. For degradation assessments, test solutions were prepared with 2.5% w/w native HA or butyric esters of HA in acetate buffer and a proper amount of bovine testicular endo-hyaluronidase. The solutions were transferred to rheometer platesa and their viscosity was measured every two minutes for two hours. The apparatus was conditioned at 37°C and the kinetic time constant was obtained by fitting the exponential decay of viscosity versus time (data not shown).
In addition, when the enzymatic concentration was equal to or higher than the concentration found in the bloodstream, HA and HA butyrate are rapidly de-polymerized (see Figure 2), indicating that modified HA would not remain in the bloodstream without being metabolized. Further, internal cytotoxicity studies by the authors did not show any toxic effects in vivo.
In vivo Antiaging Assessments
Considering the literature and applications reported, the authors sought to test the safety and efficacy of several antiaging formulations containing butyric esters of HA. The formulations tested were ordinary aqueous gels containing butyric esters of HA at concentrations between 0.1% and 1.0%, and acrylic copolymers and preservatives.
Skin irritation test: The double occlusive application of 1% hyaluronic acid butyrate was performed using 20-μL chambersb on 20 male and female volunteers (average age ~44). One cell was applied to the forearms and one to the backs of subjects to determine their skin irritation potential. Measurements were taken after cell removal at 30 min (T3) for immediate skin irritation potential, at 48 hr (T2) and at 24 hr. Visible reddening of the treatment sites indicated irritation. A five point non-parametric scale was employed to assess the severity and potential onset of reactions.30
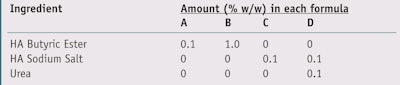
Anti-wrinkle efficacy: To assess the anti-wrinkle efficacy of HA ester butyrate from extended use,31 the authors used several noninvasive instruments to measure skin moisture, elasticity, sebum and roughness effects after the topical application of two gels employing the material at different concentrations (see Table 1, A and B) and two control gels containing HA and sodium salt (C), and HA and urea (D), respectively.
For a first set of tests, 24 female volunteers with an average age of 50 applied two test products to their faces, one on each side, twice daily for four weeks according to the randomized plan shown in Table 2; each formulation was thus used by 12 volunteers. To further examine possible differences in efficacy due to the different active concentrations,32 an additional set of tests was conducted; eight volunteers having an average age of 45.2 used only products A and B, containing 0.1% and 1.0% HA ester butyrate, respectively. Each of the products was applied to one side of the subjects’ faces twice daily for four weeks. The data collected from the first 12 volunteers was then added to the data collected from the additional eight volunteers for a total test population of 20 subjects.31
Similarly, a third set of studies with 20 subjects assessed the differences in efficacy of two formulations containing 0.1% HA ester butyrate but having different degrees of esterification—i.e., different molar fractions of butyric esters bound to an average HA repeating unit.33 In all three studies, at the beginning and after the conclusion of the fourth week of use, in a climatized room (24°C, 50% RH), instrumental measurements were taken, including: skin moisturec, elasticityd, sebume, thicknessf, luminosityg and skin roughnessh. The following key parameters were considered: arbitrary corneometric units (CU), to determine skin moisture; the viscoelastic ratio (parameter R6), Uv/Ue; maximum skin deformation (parameter R0), Uf; general elasticity (parameter R2), Ua/Uf; index of skin luminosity, L*; mean roughness (arithmetic), Ra; and maximum roughness (deep wrinkles), Rz. All of the data was statistically analyzed.
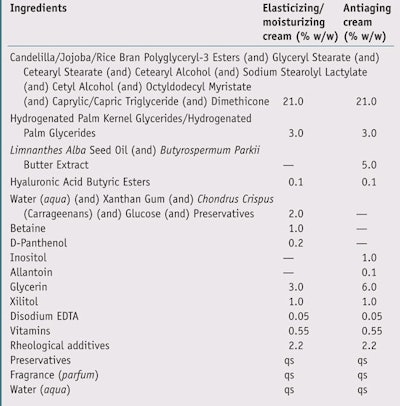
Additional in vivo tests30–35 were then carried out with two groups of 20 informed volunteers (average age ~52) to assess the efficacy of HA ester butyrate at 0.1% in two different emulsions: an antiaging cream and an elasticizing-moisturizing cream (see Table 3). Improvements in skin elasticity and moisture were assessed after extended use and compared with placebo products. At the beginning of the test and after the fourth week of use, instrumental measurements for skin elasticity and moisture were taken to assess the efficacy of the active ingredient compared with the placebo.
Results
Skin irritation test: The test products did not induce any immediate skin irritation reactions. Similarly, occlusive application for 48 hr did not induce any skin irritation reactions. Further, none of the volunteers reported any allergic reactions.
Anti-wrinkle efficacy: None of the four formulations induced significant variations in moisture corneometric values, skin sebometry or mean/maximum roughness. Moreover, no variation was found in the viscoelasticity coefficient R6. On the contrary, all gels induced a decrease in maximum skin stretchability R0, ranging between -32.2% and -40.8%, which is highly significant (p < 0.01). This means all the tested products made the skin firmer (see Figure 3).
Skin treated with 0.1% HA butyrate (product A) showed a highly significant increase in biological elasticity R2, while no significant change was noted with gels B, C or D (see Figure 4). In particular, the change induced by product A (+24.8%) was significantly different (p < 0.05) than the change induced by product D (+2.3%), which contained 0.1% HA sodium salt and 1.0% urea. This data shows that 0.1% HA butyrate enhanced skin elasticity more than a product containing 0.1% HA sodium salt and 1.0% urea, an industry standard.
All test gels induced a statistically significant increase (p < 0.05) in skin luminosity values, ranging from +1.3% to +2.7%. Moreover, results from both tests found none of the products to induce significant changes in corneometric values, viscoelasticity coefficients, or mean and maximum roughness values. Results for gels A and B concerning maximum skin extensibility were confirmed and indeed, highly significant average decreases (p < 0.01) were found with both formulas.
However, slightly different results were found concerning biological elasticity and skin thickness parameters. Besides a highly significant change in biological elasticity provided by product A (i.e. +21.1%, with p < 0.01 in the first 12 volunteers tested), an increase in the same elasticity parameter also was measured for sample B (+8.5%, p < 0.05) (see Figure 5). Statistically significant changes (-23.5%) for sebum values were found with sample gel B (p < 0.05) (see Figure 6), and skin thickness values (+ 2.2%) with sample gel A (see Figure 7). However, the changes obtained between sample gel A and B were not statistically different from one another.
Further research later proved, with the same instrumental assessment methods, that a different butyric esterification degree applied to HA, used at 0.1% in two gels, did not provide significant differences regarding elasticity, blood microcirculatory flow, luminosity, sebum and skin thickness values after extended use. In other words, the absolute amount of released butyric acid did not show significant differences.33
Tests of the emulsions containing 0.1% HA butyrate (see Table 3) showed the formulae to induce a remarkable and highly significant increase in skin moisture values (p < 0.01), +52% and +44.4%, respectively. 34, 35 In addition, a highly significant increase (p < 0.01) in biological elasticity, R2, was noted, equal to + 15.6% and +23.1%, respectively. Comparison of the tested formulas with the placebo resulted in highly significant results throughout the tests (see Figure 8).
Conclusions
HA butyrate is shown here to be a new, safe and effective ingredient for skin applications. The material induces a significant improvement in skin luminosity, maximum deformation and biological elasticity. Moreover, at low concentrations (approx. 0.1%) it increases skin thickness while at a higher concentration (approx. 1%), it helps to reduce skin sebum. HA butyrate also proved to be comparable to HA or a blend of HA (and) urea for moisturizing effects, maximum skin extensibility, viscoelastic coefficient, blood microcirculatory flow, luminosity, sebum, skin thickness and toughness. It is important to stress that the gel containing 0.1% HA butyrate yielded the best result for elasticity, R2, in particular when compared with the same concentration of HA plus urea; this difference was statistically significant.
The data also indicated greater antiaging efficacy with 0.1% HA butyrate than with other reference systems. Moreover, an increase in concentration, which was submitted to tests of higher statistical value, did not yield significant differences. Assessments carried out on two emulsions with elasticizing/moisturizing and antiaging structures confirmed that 0.1% hyaluronic ester butyrate acid significantly enhanced skin moisture and biological skin elasticity.
Therefore, the authors conclude that hyaluronic ester butyrate is a new functional ingredient with good antiaging skin effects that derive from its precursor, HA. The butyric groups have been shown to affect skin elasticity improvement by acting as messengers at low concentrations. Furthermore, hyaluronic ester butyrate is safe for cosmetic use with an innovative graft and gradual release, complemented by its skin physiology structure.
References
Send e-mail to [email protected].
1. S Inoue and Y Takahashi, Strategies for skin aging focusing on changes in DNA and tissue repair system, in Aging Skin: Current and Future Therapeutic Strategies, LD Rhein and JW Fluhr, eds, Allured Business Media: Carol Stream, IL USA (2010) pp 410–412
2. N Reinhart, GJ Cardinale and S Undenfriend, Increased turnover of arterial collagen in hypertensive rats, Medical Science 75 1 451–453 (Jan 1978)
3. B Gallot and JF Molina, Protéoglicanes, in: Actifs et Additifs en Cosmétologie, 2nd ed, MC Martini and M Seiller, eds, Tec and Doc Lavoisier, Paris (1999) pp 135-149
4. MM Rieger, Hyaluronic acid, Cosm & Toil 113 3 35–42 (1998)
5. HE John and RD Price, Perspectives in the selection of hyaluronic acid fi llers for facial wrinkles and aging skin, Patient Preference and Adherence 3 225–230 (2009)
6. R Stern et al, Hyaluronic acid and skin, Cosm & Toil 113 4 43–48 (1998)
7. W Manuskiatti and HI Maibach, Hyaluronan and Skin, Cosm & Toil 111 10 89–90 (1996)
8. I Ghersetich et al, Hyaluronic acid in cutaneous intrinsic aging, Int J Derm 33 119–122 (1994)
9. DO Schachtschabel and J Wever, Age related decline in the synthesis of glycosaminoglycans synthesized by cultured human fi broblasts (WI- 38), Mech Aging Dev 8 257–64 (1978)
10. G Sluke, DO Schachtschabel and J Wever, Age related changes in the distribution pattern of glycosaminoglycans synthesized by cultured human diploid fi broblasts (WI-38), Mech Aging Dev 16 19–27 (1978)
11. G Kogan, L Soltés and R Stern, Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications, Biotechnol Lett 29 1 17–25 (2006)
12. B Widner et al, Hyaluronic acid production in Bacillus subtilis, Applied and Environ Microbio 71 7 3747–3752 (2005)
13. M Milas, M Rinauudo and E Fouissac, Molecular weight and rheological measurements of sodium hyaluronate, Cosm & Toil 108 12 57–63 (1993)
14. M Palombo, G Delli , P Palombo and P Morganti, Ialuronan, Cosm Technol 5 2 11–14 (2002)
15. S Gariboldi et al, Low molecular weight hyaluronic acid increases the self defense of skin epithelium by induction of beta-defensin 2 via TLR2 and TLR4, J of Immunology 181 3 2103–2110 (2008)
16. European patent EPO941253B1, New butyric esters with antiproliferative activity and the pharmaceutical compositions containing them, SIGEA Srl, Trieste (IT) (May 28, 2003)
17. KN Prasad and PK Sinha, Effect of sodium butyrate on mammalian cells in culture: A review, In Vitro 12 2 125–132(1976)
18. TJ Smith, n-Butyrate Inhibition of hyaluronate synthesis in cultured human fibroblasts, J of Clinical Investigation, Inc. 79 1493–1497 (1987)
19. IS Daehn, A Varelias and TE Rayner, Sodium butyrate induced keratinocyte apoptosis, Apoptosis 11 8 1379–1390 (2006)
20. E Karna, W Miltyk and JA Palka, Butyrateinduced collagen biosynthesis in cultured fi broblasts is independent on 2 1 integrin signalling and undergoes through IGF-I receptor cascade, Molecular and Cellular Biochem 286 1–2 147–152 (2006)
21. E Karna, S Trojan and JA Palka, The mechanism of butyrate-induced collagen biosynthesis in cultured fi broblasts, Acta Poloniae Pharmaceutica Drug Research 66 3 229–233 (2009)
22. D Coradini et al, Hyaluronic acid as drug delivery for sodium butyrate: Improvement of the anti-proliferative activity on a breast cancer cell line, Int J Cancer 81 411–416 (1999)
23. D Coradini and A Perbellini, Hyaluronan: A suitable carrier for an histone deacetylase inhibitor in the treatment of human solid tumors, Cancer Therapy 2 201–206 (2004)
24. D Coradini et al, Hyaluronic-acid butyric esters as promising anti-neoplastic agents in human lung carcinoma: A preclinical study, Investigational New Drugs 22 207–217 (2004)
25. A Speranza, C Pellizzaro and D Coradini, Hyaluronic acid butyric esters in cancer therapy, Anticancer Drugs 16 4 373–379 (2005)
26. C Ventura et al, Butyric and retinoic mixed ester of hyaluronan—A novel differentiating glycoconjugate affording a high throughput of cardiogenesis in embryonic stem cells, The Journal of Biological Chemistry 279 22 23574–23579 (2004)
27. C Ventura et al, Hyaluronan mixed esters of butyric and retinoic acid drive cardiac and endothelial fate in term placenta human mesenchymal stem cells and enhance cardiac repair in infarcted rat hearts, J of Biological Chem 282 19 14243–14252 (2007)
28. R Tammi, AM Saamanen, HI Maibach and M Tammi, Degradation of newly synthesized high molecular mass hyaluronan in the epidermal and dermal compartments of human skin in organ culture J Invest Dermatol 97 126–130 (1991)
29. JR Fraser, TC Laurent, A Engstrom-Laurent and UG Laurent, Elimination of hyaluronic acid from the blood stream in the human, Clin Exp Pharmacol Physiol 11 17–25 (1984)
30. Cutaneous irritation test by patch, ISPE Study 226/06/01, Milano (2006)
31. Instrumental evaluation of moisturization, elasticity, skin blood fl ow, luminosity, sebum, pH, thickness and cutaneous wrinkledness after use, ISPE Study 213/06/01-02-03-04, Milano (2007) 32. Instrumental evaluation of moisturization, elasticity, skin blood fl ow, luminosity, sebum, thickness and cutaneous wrinkledness after use, ISPE Study 70/07/01-02, Milano (2007)
33. Instrumental evaluation of elasticity, sebum, skin blood flow, luminosity and thickness after use, ISPE Study 124/07/01-02, Milano (2007)
34. Long-term instrumental evaluation of moisturization and elasticity, ISPE Study 176/08/01-02,
Lab Practical: Using HA Butyrate
- HA butyric esters are less hydrophilic than sodium hyaluronate, thus they do not require complex swelling operations when added to water phases.
- Sodium hyaluronate butyrate dissolves in cold water under moderate stirring without forming lumps or aggregates.
- Aqueous solutions are stable and need only to be adequately preserved.
- Although the molecule is temperature-stable, it should be added to the bulk formula at no higher than 40°C during the cooling phase.