Over the past 10 years, awareness of the detrimental effects of unprotected UV exposure has increased and, as a result, consumers are seeking higher levels of protection. Regulators and the industry have responded by requiring more stringent testing and providing more complete and balanced UV protection, respectively. In relation to testing, new regulations and guidelines were introduced in Europe, the US Food and Drug Administration (FDA) proposed a new monograph in 2007, and a globally harmonized standard is currently being developed by the International Organization for Standardization (ISO).
The most significant changes within these regulations are the new requirements for UVA protection and the added test for photostability of finished sunscreen formulations. The majority of sun care products currently require SPF levels of at least 30, reaching upwards of 50+. As a result, high levels of UVA protection are required in order to make a UVA claim, as well as to meet the guidelines. To confirm that UVA efficacy guidelines have been met, conform tests are available. In addition, the photostability of these materials must be demonstrated. Fulfilling these factors proves to be challenging in the EU, and is even more so if the FDA star-rating system is to be employed.
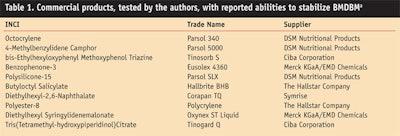
The objective of the present study was to establish photostability and UVA protection in several test sunscreen formulations. As will be shown here, butyl methoxydibenzoyl methane (BMDBM) exhibited the strongest and widest absorption curve of all the commercially available UVA filters tested. Thus, if properly stabilized, BMDBM could transfer its spectral performance benefits into higher levels of UVA protection. Since formulators seek robust UV filter combinations but are limited by cost, the authors explored several methods to photostabilize BMDBM. The performance of ten commercially available products that claim to stabilize BMDBM was investigated and their efficacy measured via two different test methods.
Materials and Methods
In a comparison study, several commercial products were evaluated for their stabilization of BMDBMa (see Table 1). These products were compared using the following two methods:
Photostability after irradiation, as described by Berset and Gonzenbach:1 The UV filters were dissolved in a mixture of 70% ethanol and a 30% mixture (1:1) of caprylic/capric triglyceride and C12-15 alkyl benzoate. Then, 2 mg/cm2 of the solution was distributed on rough glass slides and the samples exposed to 25 MED of solar-simulated light under a sun tester.b The dried films were immersed in 50 mL of methanol in an ultrasonic water bath and the UV filter concentrations were determined by HPLCc. The average deviation of the method was ± 5%.
Determination of the UVA Protection Factor (UVAPF) according to the COLIPA method (2007):2 Cosmetic emulsions were manufactured based on the emulsifier potassium cetyl phosphate, and containing different UV filter combinations to provide an SPF ≥ 30 (see Formula 1). A 0.75 mg/cm2 sample of each test formulation was applied on rough PMMA plates and the initial in vitro UVA Protection Factor before irradiation (UVAPF-0) was measuredd.
Performance targets for this study were based on the 2006 European Union (EU) Recommendation,3 specifically the minimum required level of UVA protection, and the COLIPA guidelines2 were chosen for in vitro UVA measurement and photostability testing.1 The COLIPA method has been validated and is widely adopted throughout Europe. The new EU recommendation requires that sunscreen products provide a minimum level of UVA protection that is equal to one third of the UVB protection, as measured by SPF. In the EU, the UVA performance can be measured either by in vivo Persistent Pigment Darkening (PPD),4 or by a comparable in vitro method. Subsequently, COLIPA developed an in vitro UVA test method2 as an alternative to the recommended in vivo PPD measurement (see COLIPA UVAPF testing method). This method, in addition to measuring UVA protection, also addresses the potential photo-instability of UV filters by including a pre-irradiation step.
In the present work, researchers found several effective options to stabilize BMDBM and reach photostability levels of 90–100%, even after irradiation at 25 MED.
After normalizing the absorption curve to yield and match the in vivo SPF 30, the irradiation dose was calculated by UVAPF-0 multiplied by 1.2 J/cm2 as described in the COLIPA UVAPF testing method. After exposing the samples to the estimated dose under solar-simulated lightb, the absorption was measured again. This second absorption curve contains all the potential losses due to photodegradation. After normalization of the yielded second absorption curve towards the in vivo SPF of 30, the UVAPF was calculated according to the COLIPA protocol from the fitted spectrum.
The amount of radiation required by the COLIPA method varies with the spectral performance, but typically requires, for formulations with an SPF 30 and a UVA protection factor ≥ 10, an irradiation of 10–18 MED, while formulations with an SPF 50 require approximately 15–25 MED. Since the calculations of the COLIPA method hardly call for pre-irradiations of more than 25 MED, the researchers chose to use 25 MED as the irradiation dose for the photostability experiments as a worst-case scenario, to ensure the test conditions would meet the requirements of the EU recommendation and COLIPA guidelines for most products.
There are two prominent mechanisms5 by which BMDBM can be photostabilized; i.e., triplet and singlet quenching. Photostabilizers from both of these groups were tested, combining them first in binary combinations with BMDBM (Figure 1a), then in ternary combinations (Figure 1b), in an attempt to find the optimum solution.
Initial photostability measurements were performed in films. For this, UV filter combinations were dissolved in a mixture of 70% ethanol and a 30% blend of C12-15 alkyl benzoatee and caprylic/capric triglyceridef. Then, 2 mg/cm2 samples of the solutions were applied to glass slides and irradiated with 25 MED. The UV filter concentration was determined by HPLC.
Results and Discussion
In general, triplet quenchers have been found to be the most effective at stabilizing BMDBM and in fact, among the materials tested, octocrylene consistently performed best. As shown in Figure 1a, the measured photostability of 4% BMDBM alone after 25 MED was 23%, and the researchers improved this result up to 90% with the addition of 3.6% octocrylene.
The next best performing substance was methylbenzylidene camphor, which had a comparable performance at a concentration of 4%. Not surprisingly, both substances are ideal triplet-triplet quenchers for BMDBM. Attempting to build further on the performance of the 3.6% octocrylene and 4% BMDBM mixture, researchers added an additional stabilizer and measured the resulting photostabilities. As illustrated in Figure 1b, the addition of a secondary stabilizer further increased the photostability—up to 95–100% after 25 MED.
Since octocrylene was shown to stabilize BMDBM to 90%, a significant increase above this value would need to reach 95% or more. The best performing substance to obtain this result was 4-methylbenzylidene camphor, which provided an increase of 100% with 4% filter concentration. The same performance was found with bis-ethylhexyloxyphenol methoxyphenyl triazine, with a stabilizing efficacy of 100% with 4% material. Also, a significant increase in stability of BMDBM could be achieved with 4% polysilicone-15. All other substances tested did not yield a stability level greater than the value of octocrylene alone.
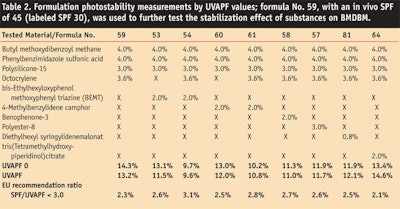
After measuring tested blends of materials, the researchers then measured the photostability of BMDBM in formulation (see Formula 1). To accomplish this, researchers chose a formulation whose in vivo SPF had previously been tested and shown, on several occasions, to provide an SPF of 30. This formulation was based on the emulsifier base potassium cetyl phosphate and incorporated various photostabilizers, as indicated in Table 2. The COLIPA method was used to determine the UVAPF before and after irradiation, and the corresponding ratio between the SPF and UVAPF was calculated to determine whether the EU recommendations were met (see Table 2).
Several compositions achieved a satisfying final UVAPF result and met the one-third ratio requirement set forth by COLIPA. For octocrylene alone, 92% of the UVAPF was retained and the SPF/UVAPF ratio was 2.3—more than sufficient to meet the target of ≤ 3.0. The addition of secondary photostabilizers did not significantly improve the UVAPF.
Using the screening data generated in the first test, researchers identified potentially interesting material combinations to reach SPF levels above 30 and introduced them into formulations. It was found that octocrylene, bis-ethylhexyloxyphenol methoxyphenol triazine, and 4-methylbenzylidene camphor all could photostabilize BMDBM sufficiently while also meeting the EU recommendation (see Figure 2 and Figure 3).
Photostability does not ensure compliance with the EU recommendation, as is illustrated by test formula 54, where perfect photostability is achieved but the UVB:UVA balance is shifted away from the 3:1 requirement. Again, it is apparent that substances providing the best stabilizing efficacy and also significant SPF contribution, since they increase the absorption curve in the UVB range, can also have an adverse impact on the final UVAPF. This is demonstrated, for example, in the case of the broad spectrum filter BEMT.
Conclusion
In the present work, researchers found several effective options to stabilize BMDBM and reach photostability levels of 90–100%, even after irradiation at 25 MED. The most effective stabilizer investigated was octocrylene, which also serves as an excellent solubilizer for BMDBM—the solubility of BMDBM in octocrylene is 13% at ambient temperature and this effect can be used to reduce the overall oil content of a formulation, thus improving the skin feel of sunscreen formulations. In addition, octocrylene, as a UVB filter, contributes to the SPF. This photostabilization system, in combination with other UVB absorbers, could enable formulators to design sunscreens with high levels of UVA protection and photostability, fulfilling the EU recommendation for sunscreen products.
References
1.G Berset and H Gonzenbach, Proposed protocol for determination of photostability. Part I: Cosmetic UV Filters, Intl J of Cos Sci (18)167–177 (1996)
2.EU Recommendation 2006/647/EC, Europa Web site, available at http://ec.europa.eu/enterprise/cosmetics/sunscreens/index_en.htm (accessed Dec 22, 2008)
3.COLIPA Project Team IV, Method for the in vitro determination of UVA protection provided by sun care products (2007), COLIPA Web site, available at www.colipa.eu/the-european-commission-recommendation-on-the-efficacy-of-sunscreen-products.html (accessed Dec 22, 2008)
4.COLIPA task force, Method for the UVA protection assessment of sunscreens based on persistent pigment darkening—testing procedure (Feb 28, 1994)
5. NA Shaath, in Sunscreens, Regulations and Commercial Development, Boca Raton, Fla. USA: Taylor & Francis Group (2005)