In technical terms, sunburn is an acute cutaneous inflammatory reaction that occurs usually between 1–24 hr after sun exposure.1, 2 The severity of the sunburn depends on two variables: the intensity or dose of light, and the length of exposure to the ultraviolet radiation (UVR). This reaction is characterized by different degrees of erythema frequently associated with pain, swelling and even the presence of blisters.2–4 Furthermore, exposure to excessive doses of solar radiation induces both acute and chronic damage to skin. However, these effects can be blocked by both UVA and UVB sunscreens. The present article therefore reviews mechanisms of UVR insult to the skin and provides sunscreen formulation suggestions for the development of sunscreen products to protect against this damage.
Photodamage and Skin Aging
Exposure to solar radiation causes sunburn, edema, premature aging expressed as wrinkles and fine lines, telangiectasia (spider veins), elastosis (leathery appearance), laxity, age spots (lentigos/freckles), seborrheic keratoses, actinic keratoses (precancerous scaly lesions) and skin cancers (basal and squamous cell carcinomas and melanoma).5–9 At the cellular level, solar radiation can produce adverse structural and functional changes in membrane proteins and lipids, chromosomal and mitochondrial DNA, and in immunocompetent factors.6–9 The source of these damaging effects is radiation in the ultraviolet range; specifically ultraviolet B (UVB), 290–320 nm; and ultraviolet A (UVA), 320–400 nm.5–9
UV rays penetrate the skin to different depths and as a result, elicit different biologic effects (see Table 1).5, 8, 9 The skin is composed of three layers including the outer, dead stratum corneum layer; the living epidermis, which is metabolically active and continuously replaces the stratum corneum; and the thick dermis, which is the supporting layer consisting of connective tissue. UVR of approximately 290–320 nm (UVB) penetrates the stratum corneum and upper part of the epidermis, and is sufficiently energetic to cause severe burning (erythema) of the skin, especially in fair-skinned individuals. Chronic exposure to high energy UVB causes mutations in the DNA, leading to skin carcinomas.
UVA from 320–400 nm penetrates the epidermis and begins to penetrate the dermis. In the lower epidermis are actively dividing cells such as basal keratinocytes and other immune-modulating cells, i.e., Langerhans or dendritic. These cells are vulnerable to UV, and damage to them causes immunosupression.6, 7 The lower epidermis also contains melanocytes, which generate the melanin pigment responsible for skin color. Exposure to UVA rays will stimulate the formation of melanin, thus producing a tan that protects the skin from immediate sunburn. Although UVA rays are of a lower energy than UVB rays, they can extend into the growing and proliferative basal layers of the epidermis and also into the dermis, contributing to photodamage and photoaging.10–13 They may also potentiate the carcinogenic effects of UVB.4 Thus, UVA and UVB substantially contribute to chronic skin damage, and continued exposure to UVR accelerates aging in skin.10–15
Wrinkling, elastosis and pigment alterations (solar lentigines) are the most common consequences of long-term sun exposure.15–20 In relation, in the last two decades, the pathogenesis of UVR-induced collagen damage has been well understood and characterized.21 For instance, it is known that UVR exposure significantly upregulates the synthesis of several types of collagen-degrading enzymes known as matrix metalloproteinases (MMPs). It also has been shown that chronic UVB irradiation of the dorsal skin in hairless mice forms wrinkles and that chronic UVA irradiation forms mainly sags.22, 23 In other animal models, chronic UVA irradiation increased skin elastase activity and destroyed the three-dimensional structure of dermal elastic fibers, decreasing skin elasticity similarly to UVB irradiation. This action could be blocked by both UVA and UVB sunscreens.24
Sunscreens
The studies cited above, among countless others, lead to the conclusion that protective and preventive measures must be taken to guard against the damaging effects of the complete spectrum of UV light on skin. Furthermore, sunburn protection measures should be taken early and continued throughout life so as to prevent the cumulative effects of excessive sun exposure. The best methods of sunburn protection are avoidance during peak hours; protective clothing including hats, sunglasses and light-colored, tightly woven garments; and the use of topical products containing chemical filters (sunscreens) that broadly absorb UVR across the short and long wavelengths.25–40
Sunscreens are designed specifically to interfere with the skin’s normal response to solar radiation. They accomplish this by absorbing, reflecting or scattering UVR. The greatest sunscreen efficacy is achieved by combining active ingredients to provide the widest range of sun protection. These actives act as a series of filters to remove or cancel out the most harmful and damaging rays of the sun. Other characteristics, such as water resistance, contribute to the overall effectiveness of the product and take into account the habits and practices of consumers.
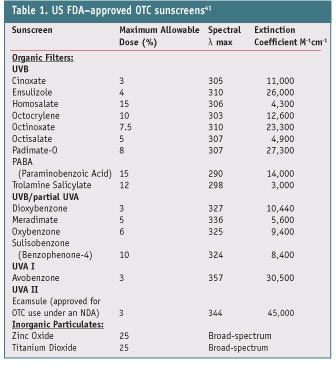
Sunscreens on the current global market primarily contain UVB filters and afford varying degrees of sun protection beyond UVB (SPF values) and into the UVA region (PFA values). In light of recent findings regarding the effects of UVA, broad-spectrum coverage is essential, and countries with the most aggressive sun protection initiatives such as Australia recognize this need.32 In the United States, 15 UVB filters including 13 organic and two inorganic particulates are approved that absorb in the region from 280–320 nm.41 These ingredients along with their allowed amounts, maximum wavelength and extinction coefficient are shown in Table 1. This list of UV filters, with the exception of avobenzone and micronized forms of zinc oxide and titanium dioxide, reflects old UV filter designs that were state-of-the-art research for the early 1970s. Before 2005, only one UVA filter was approved under the US Federal Monograph: avobenzone. Avobenzone filters the narrow region of UVR called UVA I (340–400 nm); in other parts of the world, several additional UVA filters have been and are still used to absorb more broadly in this UVA region (see Table 1).
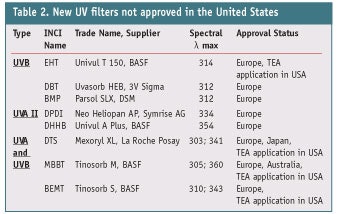
In 2005, another UVA sunscreen was approved under a New Drug Application (NDA) in the United States: ecamsule. However, since there are already four commercial products combining this ingredient with other approved sunscreens, this sunscreen cannot be used by other manufacturers until the patent coverage expires-after which another company can submit an abbreviated NDA seeking approval for similar products. Ecamsule filter absorbs optimally at 344 nm and broadly in the shorter UVA II region of 320–360 nm. Outside of the United States, many other sunscreens are available, including bis-ethylhexyloxyphenol methoxyphenyl triazine and drometrizole trisiloxane, which absorb strongly in the UVA II region. Tucninda et al. published the latest review of emerging sunscreen technologies and their benefits42 and with the exception of encamsule, none of the remaining sunscreens in Figure 1 absorbs to any appreciable extent in this region.
The Ultimate UV Filter
While broad-spectrum coverage is essential to optimum skin protection, no one sunscreen filter can provide both high protection along with broad-spectrum coverage. This is due to a combination of factors: local regulatory limits on levels allowed in formulations; the stability of given ingredient combinations; the esthetics of the product—i.e., too heavy or greasy feeling; and the absorption limitations of any single ingredient. Envisioning the ultimate sunscreen, Shaath43 described the ideal filter as follows.
First, it should absorb harmful UVR in the 280–400 nm region. To achieve this, two or more sunscreens that filter in the 280–320 nm (UVB) region and the 320–400 nm (UVA) region may be necessary. In addition, the individual filters should possess high molar extinction co- efficients at their maximum wavelength. Values exceeding 25,000 would be the best to provide the maximum possible protection for the dose of sunscreen used in the formulations. Of note are newer molecules designed in Europe with multiple chromophores that have unusually high extinction coefficients.
Also, the ideal filter should have excellent photostability and be photochemically inert. The leading UVA sunscreen in the United States, avobenzone, is known to be photo-unstable. If isomerization such as cis-trans or keto-enol is possible in the molecule, then the degradation quantum yields should be low, indicating that isomerization is reversible. Inorganic particulates are being commercially produced with the least amount of photochemical reactivity possible. Therefore, the type of mineral, its specific coating and the type of dispersant must be carefully selected and studied in the formulation.
The ideal UV filter also should have good solubility in the emollients selected. Solid sunscreens such as the benzophenones, avobenzone and camphor derivatives require special techniques and excipients to solubilize in formulations so they do not crystallize out on the skin. The maximum wavelength and molar extinction coefficient also should not be affected by solvents or excipients. In water-resistant formulations, sunscreens should be insoluble in water, although water-soluble sunscreens also play a role in sun protection, such as in hair preparations or when SPF boosting is required. In addition, the sunscreen should not be irritating to skin and eyes, comedogenic, sensitizing or phototoxic.
Compatibility with cosmetic vehicles and ingredients as well as packaging is also important, as is its ease of formulation and manufacture. Furthermore, various filter combinations should be patent-protected.
Additional considerations include the fact that UV filters constitute a significant portion of the cosmetic formulation, sometimes as much as 15%, and may impart moisturizing advantages-although they should not discolor the skin or clothing, or produce off-odors upon application. The ideal UV filter should be available isometrically pure, chemically stable during long- term storage, and chemically inert to other cosmetic ingredients. Finally, it should be cost-effective and approved worldwide by the official regulatory agencies with the fewest restrictions on levels used or combinations.
Formulae Development The efficacy of certain combinations of UV filters will vary with the formulation base, but the generally accepted rule of thumb is to use 7.5% octinoxate, 3% oxybenzone and 3% avobenzone to achieve an SPF 15. Higher sunscreen content will produce higher SPFs. In addition, solvents used in the system may have a positive or negative effect on the λ max achieved, known as the solvent-shift effect. For example, phenethyl benzoate may shift the UVA absorption curve to the right, resulting in a higher UVA reading. Considering these variations, the formula vehicle and form are discussed here.
Emulsions: The most popular vehicle used for sunscreens is the emulsion because it offers the broadest formulation advantages and versatility. Klein and Palefsky44 reported that emulsions are best because they exhibit good performance in areas important to consistently high SPFs such as uniformity, thickness and nontransparency of film, and minimum ingredient interactions.
Emulsions facilitate the incorporation of sunscreens, which are usually oils that can be easily emulsified. Emulsions also can be formulated to contain large amounts of water, making them more cost-effective. It is also well-known that emulsions give the skin a smooth, silky feel without being greasy and can accommodate a wide variety of raw materials. On the negative side, emulsions are typically thermodynamically unstable, with the exception of spontaneously forming microemulsions. Additionally, emulsions present a perfect medium for microbial contamination and require preservatives.
For sunscreen formulations, w/o emulsions are best45 because by design, they are water-resistant and provide greater efficacy (a higher SPF) for the same concentration of sunscreen activities, compared with o/w emulsions. However, water-resistance is not necessary for formulation types such as makeup that contains sunscreens and offers daily protection from incidental UV light. Klein and Palefsky also explain44 that since most sunscreens are soluble in the oil phase, and in w/o emulsions, the oil phase is continuous, when the formula is applied to skin, agglomeration does not need to occur. Therefore, a uniform film is produced during spreading, along with a high SPF.
Liquid crystals: Another alternate formulation approach discussed by Klein and Palefsky is the use of emulsifiers that promote the formation of liquid crystals.44 It is well-known that emulsions are often stabilized by liquid crystals. Generally, these liquid crystals are lamellar in structure and either form a gel network in the external phase or surround the oil droplets as layers. In both cases, the liquid crystal structure reduces the tendency to coalesce due to the high viscosity of the lamellar structure, facilitating high emulsion stability. Another advantage is that such emulsions are not very hydrophilic and provide water-resistant characteristics for sunscreen formulations. Their lipoidal characteristics facilitate adhesion to the hydrophobic/lipidoidal skin surface and thus enable the formation of a uniform film.
Oils: Oils are another generally well-accepted historic sunscreen vehicle that are easily formulated. Again, Klein and Palefsky44 enumerate other benefits, such as only one phase being necessary, so oils have excellent product stability. And because most sunscreen actives are lipoidal in nature, they are soluble in oils—which makes their manufacture more straightforward, compared with emulsions; in fact, most can be prepared at room temperature. Further, they are easy to apply to skin, spreading quickly and uniformly to cover a large area.
However, although oils have excellent spreadability, this often results in a thin, transparent sunscreen film, which will have a lower SPF. It is well-known that sunscreen oils yield the poorest SPF performance of any vehicle, which can be explained by looking at the interactions between the sunscreen (nonpolar esters) and the very nonpolar oil vehicles. Nonpolar oils such as mineral oil can cause the position of the UV curves to shift to shorter wavelengths, < 290 nm, limiting the benefit of the sunscreen from the claimed SPF. Klein and Palefsky44 attributed this shift to stabilization of the ground state by the nonpolar vehicle.
Further, oils are limited as far as packaging to minimize interactions and cost since this anyhydrous system contains no water to lower the cost of expensive raw materials. Sunscreen oils are therefore one of the most expensive systems found.
Formula types: Other types of formulations, detailed elsewhere,44 are gaining popularity but many suffer from some of the same issues for achieving high SPFs and reducing cost. While sun care products have historically been marketed in cream, lotion and wipe forms, the popularity of spray forms is gaining momentum in the United States, especially for anhydrous systems. Specific ingredients and their effects on UVB and UVA protection are becoming more important, especially in light of recent worldwide attention by regulatory authorities. Products that deliver multifunctional benefits such as water-resistance, rub-resistance, sprayability and thickening, all with esthetically desirable feel, are becoming popular. Such products include acrylic acid/VP crosspolymer, which is a thickener and suspending agent for inorganic materials such as zinc oxide and inorganic pigments; and VP/dimethylaminoethylmethacrylate copolymer or butylated PVP, a water-resistance agent applicable for sprays with a nice feel.
With color cosmetics in particular, to impart UV protection, chemists must bear in mind that different skin types will require different sunscreens, so adequate tests must be performed on color cosmetics to determine the optimal blend of sunscreens for use on all skin types. For example, Formula 1 is a sunscreen foundation with good spreadability that is easily prepared; however, the use of titanium dioxide, magnesium oxide, zirconium oxide, zinc oxide and talc will produce an ashy appearance on darker skin. The solution would be to use a formulation similar to Formula 1. The ashy appearance is eliminated by using a translucent mica, titanium dioxide and iron oxides. The use of the lithium stearate helps the powders adhere to skin. An SPF of approximately 17 is achieved by the microfine titanium dioxide.
Formulating pigmented emulsions such as these with one sunscreen is advantageous in that they have a high photostability profile. The downside is their hydrophobic effect, which may be unstable for the length of a Drug Stability Protocol. In US patent 5,585,090, however, Yoshioka et al.46 showed that combining a metal oxide flake and a UV absorbent-encapsulated polymer resin enhanced SPF efficacy and maintained stability, and also required smaller amounts of both components in formulations. The inventors provided examples of polymer resins, including vinyl polymers, acrylic resins, polystyrenes and some others; UV resins included salicylic acid, benzophenone derivatives and aminobenzoic acid derivatives, and the metal oxides included zinc oxide, titanium dioxides and zirconium oxides.
Sunscreen Testing
Due to the high costs of launching a sunscreen product, chemists must conduct preliminary tests to ensure ease of transition from product development to the consumer. Several tests typically are performed, including:
- At least eight weeks of in-house stability testing of the finished formula in the final package, including drug substance and physical and microbiological preservation testing;
- A 10% production batch of at least three batches to qualify and validate the method and equipment used for the manufacturing;
- Twelve weeks of accelerated over-the-counter drug substance stability testing under the auspices of cGMPs. The lab will develop and validate the method used to determine the sunscreen active in the formula and this test will determine a tentative two-year expiration date, which is required by the 21CFR part 211.137 and 211.166;47 and
- A 36-month controlled room temperature stability test according to US Food and Drug Administration-approved guidelines. This test includes physical testing as well as the assaying of actives at scheduled intervals; such as 0, 1, 2, 3, 6, 9, 18, 24 and 36 months. Where applicable, the inclusion of preservative efficacy testing is also highly recommended.
Finally, tolerability testing as well as tests for photoallergy and phototoxicity (if the sunscreen contains a suspected photo-allergen) and a Draize human repeat insult patch test for irritation and sensitization are completed.
Summary
Although no association has been made between sunscreen use and the development of malignant melanoma, the mechanisms of UVR insult and resulting photodamage to the skin are known. With new and more effective sunscreens coming out on the market, it is anticipated that the benefit of using modern sunscreens will be shown in future research and epidemiology studies. However, the habits and practices of the consumer must favor use of sun protection measures. This is where the formulation of effective and esthetically pleasing sunscreen products to protect against damage becomes crucial and will shape the future landscape of the industry.
References
- CR Taylor and AJ Sober, Sun exposure and skin disease, Ann Rev Med 47 181–191 (1996)
- PG Norris, RW Gange and JLM Hawk, Acute effects of ultraviolet radiation on the skin, in: TB Fitzpatrick, AZ Eisen, K Wolf, IM Freedberg, KF Austin, eds, Dermatology in General Medicine, McGraw-Hill, NY (1993)
- KB Pandolf et al, Human thermoregulatory responses during heat exposure after artificially induced sunburn, Am J Physiol 262 610–616 (1992)
- NA Soter, Acute effects of ultraviolet radiation in the skin, Semin Derm 9 11–15 (1990)
- N Shaath, The chemistry of sunscreens, in: N Shaath, ed, Sunscreens 3rd edn, Taylor and Francis, NY (2005)
- F Urbach and R Grange, eds, The Biological Effects of UVA Radiation, Praeger, NY (1986)
- SE Ulrich, Mechanisms underlying UV-induced immune suppression, Mutat Res 571(1–2) 185–205 (2005)
- L Rhein and S Harripersad, Sunscreens, in surfactants in personal care and decorative cosmetics, in: L Rhein, M Schlossman, A O’Lenick and P Somasundaran, eds., Surfactants in Cosmetics Series, 3rd edn, ch 15, Taylor & Francis, FL (2006)
- KC Farmer and MF Naylor, Sun exposure, sunscreens and skin cancer prevention: A year-round concern, Ann Pharmacol 30 662–673 (1996)
- LH Kligman, Photoaging. Manifestations, prevention and treatment, Derm Clin 4 517–528 (1986)
- WJ Sams, Sun-induced aging. Clinical and laboratory observations in humans, Clin Geriatr Med 5 223–233 (1989)
- CE Griffiths, The clinical identification and quantification of photodamage, Br J Derm 127(suppl 41) 37–42 (1992)
- A Engel, ML Johnson and SG Haynes, Health effects of sunlight exposure in the United States, Arch Derm 124 72–79 (1988)
- LH Kligman and MA Kligman, The nature of photoaging: Its prevention and repair, Photoderm 3 215–227 (1986)
- AR Young, Cumulative effects of ultraviolet radiation in the skin: Cancer and photoaging, Semin Derm 9 25–31 (1990)
- A Fourtanier and C Berrebi, Miniature pig as an animal model to study photoaging, Photochem Photobiol 50 771–784 (1989)
- SJ Moloney, SH Edmonds, LD Giddens and DB Learn, The hairless mouse model of photo-aging; Evaluation of the relationship between dermal elastin, collagen, skin thickness and wrinkles, Photochem Photobiol 56 505–511 (1992)
- S Imayama et al. Ultraviolet B irradiation deforms the configuration of elastic fibers during the induction of actinic elastosis in rats, J Derm Sci 7 32-38 (1994)
- J Leyden, What is photoaged skin? Eur J Derm 11(2) 165–167 (2001)
- RE Herschenfeld and BA Gilchrest, The cumulative effects of ultraviolet radiation on the skin: Photoaging, in: JL Hawk, ed, Photodermatology, Chapman and Hall, London 69–87 (1998)
- L Baumann, Skin aging and its treatment, J Pathol 211 241–251 (2007)
- DL Bissett, DP Hannon and TV Orr, An animal model of solar-aged skin: Histological, physical and visible changes in UV-irradiated hairless mouse skin, Photochem Photobiol 46 367–378 (1987)
- K Tsukahara et al, Ovariectomy is sufficient to accelerate spontaneous skin aging and to stimulate ultraviolet irradiation-induced photoaging of murine skin, Br J Derm 151 984–994 (2004)
- K Tsukahara et al, The effect of sunscreen on skin elastase activity induced by ultraviolet-A irradiation, Biol Pharm Bull 28(12) 2302–2307 (2005)
- SQ Wang et al, Ultraviolet A and melanoma: A review, J Am Acad Derm 44 837–846 (2001)
- International Agency for Research on Cancer, IARC monograph on the evaluation of carcinogenic risks to humans: Ultraviolet radiation, No. 55, IARC, Lyon (1993)
- B Staberg et al., Carcinogenic effects of sequential artificial sunlight and UVA irradiation in hairless mice, Arch Derm 119 641–643 (1983)
- B Staberg et al., The carcinogenic effect of UVA irradiation, J Invest Derm 81 517–519 (1983)
- H van Weedlen, F Gruijl and J van der Leun, Carcinogenesis by UVA with an attempt to assess the carcinogenic risks of tanning with UVA and UVB, in: F Urbach and R Gange, eds, The Biological Effects of UVA Radiation, Praeger, NY (1986)
- N Passchier and B Bosnjakovic, eds, Human exposure to ultraviolet radiation: Risks and regulations, Exerpta Medica International Congress Series, Amsterdam 744 (1987)
- EA Holly, DA Aston, RD Cress, DK Ahn and JJ Kristiansen, Cutaneous melanoma in women, Am J Epidemiol 141 923–933 (1995)
- AD Woodhead, RB Setlow and M Tanaka, Environmental factors in nonmelanoma and melanoma skin cancer, J Epidemiol 9 S102–S114 (1999)
- S Seite et al., Mexoryl SX: A broad absorption UVA filter protects human skin from the effects of repeated suberythemal doses of UVA, J Photochem Photobiol 44 69–76 (1998)
- S Seite et al, A full UV spectrum absorbing daily use cream protects human skin against biological changes occurring in photoaging, Photoderm Photoimmunol Phototmed 16 147–155 (2000)
- BK Armstrong and A Kricker, How much melanoma is caused by sun exposure? J Clin Epidemiol 395–401 (1993)
- R Stern, M Weinstein and S Baker, Risk reduction for nonmelanoma skin cancer with childhood sunscreen use, Arch Derm 122 537–545 (1986)
- RP Gallagher et al, Broad spectrum sunscreen use and the development of new nevi in white children: A randomized controlled trial, JAMA 283 2955–2960 (2000)
- SC Thompson, D Jolley and R Marks, Reduction of solar keratoses by regular sunscreen use, New Eng J Med 329(16) 1147–1151 (1993)
- MF Naylor, A Boyd, SW Smith, GS Cameron, D Hubbard and KH Neldner, High sun protection factor sunscreens in the suppression of actinic neoplasia, Arch Derm 131 170–175 (1995)
- A Green et al, Daily sunscreen application and beta carotene supplementation in prevention of basal cell and squamous cell carcinomas of the skin: A randomized controlled trial, Lancet 354(9180) 723–729 (1999)
- Department of Health and Human Services, US Food and Drug Administration, Sunscreen drug products for over the counter human use; Final monograph, Federal Register 64(98) 27666–27693 (May 21, 1999)
- C Tuchinda, H Lim, U Osterwalder and A Rougier, Novel emerging sunscreen technologies, Derm Clin 24 105–117 (2006)
- N Shaath, The chemistry of sunscreens, in: N Shaath, ed, Sunscreens 3rd edn, Taylor and Francis, NY (2005) 44. K Klein and I Palefsky, Formulating sunscreen products, in: N Shaath, Sunscreens 3rd edn, Taylor and Francis, NY (2005)
- CF Garland, F Garland and ED Gorham, Rising trends in melanoma, An hypothesis concerning sunscreen effectiveness, Ann Epidemiol 3 103–110 (1993)
- US Pat 5585090, Cosmetics having sun