Silicones don’t grow on trees, and they never will be organically certified. However, will they ever be accepted by the natural and organic market? And can silicones be green? A dark cloud still hovers over this versatile and unique class of ingredients, cast by the private certifiers of natural cosmetics, global regulatory bodies, NGOs and mass media. These organizations and vocal critics hope to influence concerned consumers, such as those following lifestyles of health and sustainability (LOHAS)1—who are estimated to represent 13–19% of total U.S. adult consumers. They also seek to influence the formulators who endeavor to satisfy these consumer needs.
None of silicone’s derivative dimethicone compounds have been found in nature; ergo, the Natural Products Association’s (NPA’s) standards2 prominently prohibit “synthetic silicone ingredients.” Likewise, the NSF/ANSI 305 Standard for Personal Care Products3 provides a list of prohibited ingredients that includes both cyclopentasiloxane (D5) and dimethicone.
Across the Atlantic, one will not likely find Ecocert silicones or those certified to NaTrue standards. And industry expert Judi Beerling states, in her introductory lesson for the online Cosmetics & Toiletries “Developing Natural Cosmetic Formulations” course,4 that silicones are prohibited synthetics in natural and organic certification organizations. However, those who go directly to retailer standards for natural products, such as Whole Foods Market,5 will find D5 is accepted in “Regular Body Care” but not “Premium Body Care.” Dimethicone is acceptable for both categories but its use in Premium status products is under review, as of the writing of this paper.
Safety Data
Formulators may want to evaluate the data themselves for both safety and environmental aspects to make their own judgments. For safety, one can refer to Cosmetic Ingredient Review (CIR) publications, which are now available at www.cir-safety.org/ingredients. One CIR report from 2003 covers dimethicone and a wide assortment of derivatives,6 while a 2011 report covers the volatile cyclics, including D5.7
The CIR’s expert panel of toxicologists has judged dimethicones and cyclomethicones as “safe as used” in personal care products. In addition, the Scientific Committee on Consumer Safety (SCCS) in the European Union (EU) has reviewed the data and concluded that “cyclomethicone (D4, D5) does not pose a risk for human health when used in cosmetic products.”
Environmental Effects
The SCCS deferred to Health Canada for comment on environmental effects, which were inconclusive. In 2008, Health Canada had cautioned that cyclic silicones have or may have a harmful effect on the environment. However, in the October 2011 report to the Minister from the Siloxane D5 Board of Review, the board concluded that D5 did not present a risk to the environment.8
As a point of interest, in the dry cleaning industry, cyclopentasiloxane (D5) is promoted as an environmentally friendly replacement solvent for the chlorinated organic perchloroethylene typically used. The Green Earth Cleaning group licenses a non-toxic dry cleaning that uses liquid silicone, in this case D5, made from silica or sand. The implication is: What could be more natural? In relation, the Green Earth Cleaning Fact sheet9 highlights D5’s benefits, including that it is not a volatile organic compound (VOC). In addition, the U.S. Environmental Protection Agency (EPA), via its Significant New Alternatives Policy (SNAP) program, is reviewing D5 an alternate to other ozone-depleting materials.
Further, an environmental fate chart by General Electric indicates the breakdown of D5 in air by photolysis, in landfill incineration and soil hydrolysis to silica (SiO2), CO2 and water.10 These environmental breakdown routes are echoed in Dow Corning’s summary sheet11 for dimethicone or polydimethylsiloxane (PDMS), which indicates that the primary destination for non-volatile silicone fluid residues is in sewage sludge. This sludge is either incinerated—resulting in carbon dioxide, water vapor and silicon dioxide end products—or degraded in the soil as the same compounds. According to the summary, in soil, “the initial step is [being] catalyzed by clay, and involves a combination of random scission to shorten the polymer chain. The principal degradation product is dimethylsilanediol (DMSD), which can ultimately degrade back to carbon dioxide, silicon dioxide and water.”
Silicone Alternatives
Silicones, at least simple and volatile cyclic and straight chain dimethicones, may be safe as used in cosmetics. They may also generate an environmental impact of low concern. However, formulators may still seek alternates for marketing initiatives arising from concerns noted previously in this paper or misguided consumer perceptions. But replace them with what?
According to Blakely and Van Reeth,12 silicones in personal care can be divided into five families. Briefly, these are: 1) volatile cyclomethicones; 2) linear dimethicones—i.e., the range of fluids generically known as silicones; 3) blends of higher molecular weight dimethicone gums and trimethylsiloxysilicate resins dispersed in low viscosity silicones; 4) dimethicone crosspolymers—elastomers of varying degrees of cross-linking and composition, which come as neat powders or swollen suspensions in cyclomethicone, dimethicone and blends thereof; and 5) functional silicones. The latter is a growing range of alkyl-substituted methicones, alkoxylated derivatives, aminosilicones and virtually any hybrid chemistry imaginable.13
In considering silicone alternatives, the market typically confines itself to the first two types—volatile cyclic (essentially D5) and linear dimethicone fluids. Purveyors of such silicone substitutes focus on the properties of volatility, viscosity and skin feel. Therefore, the first generation of silicone replacements consists of emollients (chemically classified as hydrocarbons) or esters and ethers.
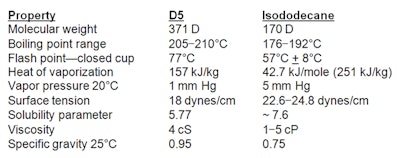
Hydrocarbons: A prominent blend of hydrocarbons modeled after D5 to mimic its feel and volatility is an isohexadecane, isododecane and C13-15 alkane blend from Prespersea.14 As a direct replacement, this patented blend of branched and straight chain alkanes is said to provide “superior spreading and lubricity properties with unique volatility and solubility characteristics.” To understand the differences between the volatile silicone D5 and the volatile hydrocarbon isododecane, refer to Table 1.
Sasol’s range of liquid linear hydrocarbonsb can be placed in the same category. Made from unspecified renewable vegetal resources, the products are claimed to be “natural-based replacements for cyclomethicone D5, or petrochemical isoalkanes.” They are Ecocert-listed and biodegradable, according to the company. Likewise, coconut alkanes derived by the “complete reduction and hydrogenation of a mixture of fatty acids derived from Cocos nucifera (coconut) oil”c are completely volatile and present drier, more raspy emollients than D5. Grant Industries supplies these coconut alkane hydrocarbons, including an option with coco-caprylate/caprate.15
Emollients: Similar to esters and ethers, an olive-based emollientd from Soliance provides a silky feel and sheen to the skin, which absorbs quickly but leaves a distinctly perceptible and light lipid film. As a visual aid, in comparing this emollient with both cyclomethicone and 20 cs dimethicone fluid, the company presents two sensory maps with mineral oil as a negative benchmark. The new emollient aligns closely with the low viscosity silicone fluid, both neat and as a 12% oil phase in a test emulsion. Thus, the ingredient is said to impart “softness to the formulation with a non-greasy after-feel,” with reduced soaping on rub-in. A similar entry from Clariante also makes use of olive feedstocks in its light, silky-feeling emollient.
Esters and Ethers: Targeting the silky feeling of D5 without its volatility is a diverse group of esters and ethers promoted for their spreading and absorption into the skin. One of the earliest on the scene was based on milk thistlef, and consists of a preponderance of unsaturated omega-6 fatty acids that support vitamin F and essential fatty acid claims for skin benefits. Surprisingly, the single seed of dried fruit milk thistle contains up to 30% lipids, which, when trans-esterified to ethyl esters, are dramatically reduced in viscosity to < 5 cps and slightly reduced in surface tension to 30.5 dynes/cm—closer to the target silicones. Their spreadability on skin mimics D5 with apparent skin absorption that is quick enough to give the illusion of volatility.
Cognis, now a part of BASF, introduced a rangeg of light emollients to meet the need for cyclomethicone replacements. The line includes high-spreading, nonvolatile emollients that mimic the feel of D5 before it leaves the skin surface, giving the sensation of penetration. Dicaprylyl ether, a vegetable-derived material, is stable against hydrolysis, and dicaprylyl carbonate is recommended for sunscreen emulsions and pigment dispersions, as it is an effective solvent for crystalline sunscreen actives and reduces the greasy feel of high active preparations. Also, the company’s coco-caprylate has a spreading value of 1,300 mm2/10 min—much higher than typical emollients such as caprylic/capric triglyceride. Lastly, caprylyl caprylate/caprate is an enzyme catalyzed ester for sensitive skin sourced from natural feedstocks.
A heptyl undecylenateh ingredient from INOLEX is derived from castor oil, and is a light, dry emollient designed as a natural alternative to cyclomethicone and mineral oils. This material is also reported to reduce the greasy feel of heavier emollients.
Croda also has introduced emollientsj to replace cyclomethicone in skin care. The company’s PPG-3 benzyl ether myristate is said to perform better than silicones in shine and feel attributes—benefits for which silicones are known. The benzyl moiety may be what gives the material a high refractive index, which increases shine and emulsion opacity/whiteness. This moiety also improves the material’s solvency for the sunscreen benzophenone. A related ester, PPG-3 benzyl ether ethylhexanoate, compares favorably to D5 in sensory tests and shows better sunscreen solvency than D5.
Finally, another unique emollient is PPG-3 isostearyl methyl ether, which employs the company’s ether chemistry to provide a wider pH stability profile with lower viscosity and polarity, in turn resulting in high spreading on the skin for a light feel.
Conclusion
The target at which these organic emollients are aiming is D5, and to a lesser extent, low viscosity silicone fluids. Many of these alternative technologies meet the mark if the industry really wants to replace silicones in personal care. However, a second look at why silicones are being replaced and whether the right properties are being duplicated is advised. In the long run, the industry will need to view all its ingredients for broader sustainability issues, beyond safety and environmental impact. A look at energy use and carbon footprint is also recommended, as well as the use of natural resources that may compete with food production and other more critical and basic needs.
References
- www.lohas.com/about (Accessed Aug 29, 2014)
- www.npainfo.org/App_Themes/NPA/docs/The%20Natural%20Standard%20010214.pdf (Accessed Aug 29, 2014)
- www.nsf.org/services/by-industry/food-safety-quality/organic-and-specialty-certifications/personal-care-nsf-ansi-305 (Accessed Aug 29, 2014)
- J Beerling, Introduction lesson #1. Developing natural cosmetics formulations, Complete Cosmetic Chemist Training Program (discontinued), Allured Business Media, Carol Stream, IL USA (2012)
- www.wholefoodsmarket.com/about-our-products/premium-body-care-standards (Accessed Aug 29, 2014); “regular” body care standards available upon request to Whole Foods
- Safe as used, available at www.cir-safety.org/sites/default/files/S-safeasused062013.pdf (Accessed Aug 29, 2014)
- Dimethicone, Intl J Toxicol 22(suppl 2) 11-35 (2003)
- M DePoortere, Silicones: Rebalancing the debate, Personal Care 109-110 (Feb 2012)
- c.ymcdn.com/sites/www.greenearthcleaning.com/resource/resmgr/docs/gecfactsheet5.10.pdf (Accessed Oct 7, 2014)
- c.ymcdn.com/sites/www.greenearthcleaning.com/resource/resmgr/docs/environmentalfatechart_000.pdf (Accessed Oct 7, 2014)
- www.dowcorning.com/content/about/aboutehs/EHSPortalFiles/Silicones_in_the_Environment-Dow_Corning.pdf (Accessed Aug 29, 2014, after entering an e-mail address)
- J Blakely and I Van Reeth, Silicones: A key ingredient in cosmetic and toiletry formulations, in Handbook of Cosmetic Science and Technology, 2nd edn, Marc Paye et al, eds, CRC Press/Taylor & Francis, Oxford, UK (2006)
- AJ O’Lenick, Jr. Silicone chemistry, in Beginning Cosmetic Chemistry, 3rd edn, R Schueller and P Romanowski, eds, Allured Business Media, Carol Stream, IL USA, ch 14 (2009)
- US Pat 8,506,974, Silicon-free hydrocarbons providing aesthetic volatility, Laba et al, assigned to Presperse (Aug 13, 2013)
- www.personalcaremagazine.com/print.aspx?story=6378 (Accessed Aug 29, 2014)