Silicone polymers have experienced tremendous growth in the personal care market. Known since the 1860s and commercialized with the pioneering work of Rochow1 since the 1940s they are now present in almost every personal care product category. The reason for this expansion is twofold: first, increasing classes of compounds have been developed; and second, the formulator has learned how to incorporate them efficiently into formulations. The continued development and application of silicone compounds for personal care requires the use of amphiphilic silicones—silicones with two or more groups that, in their pure form, are insoluble in one another.
These surface active or surfactant silicones move to the surface of the oil phase in which they are contained, much like sodium laureth sulfate moves to the surface of water, and lower the surface tension, allowing for easier spreading on the skin and hair. For most alkyl silicones, a concentration of 1% w/w lowers the surface tension. This makes them effective in providing a silicone-like feel to oils, most interestingly natural oils such as olive oil. If the concentration is increased to between 5-10% by weight, micelles form, providing a thixotrophic gel and change in aesthetics.
Why Silicone?
Silicones are used in formulations due to their low surface tension, which contributes to a unique feel. Silicone compounds have a surface tension of approximately 20 dynes/cm2. In contrast, oils have a surface tension in the range of 30 dynes/cm2 while water is around 72 dynes/cm2. Therefore, silicone compounds having both silicone-soluble and silicone-insoluble groups provide formulators with functional surface active agents to overcome these differences in surface tension.
The silicone-insoluble components can include oil soluble groups such as alkyl silicones as well as water soluble groups, i.e., dimethicone copolyols. This basic chemistry has been known for some time; however, new materials have been developed to impart functionality at low use levels (below 1%). These materials, referred to as high definition polymers, are precisely tuned to certain molecules to impart specific benefits in a formulation.
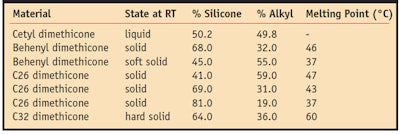
Alkyl silicones are one class of compounds that can be made into high definition polymers; other classes include dimethicone copolyols and silicone resins. The ratio of silicone to alkyl in these materials determines the degree of occlusivity of the film and the clarity of the alkyl dimethicone in natural oils. The higher the percentage of silicone in the alkyl dimethicone, the more breathable the film and the more opaque the resulting gel. The ratio of silicone to alkyl in the alkyl dimethicone also determines the hardness it provides.
Structure and Function
The structure of a polymer determines its function. In Figure 1, R is the alkyl or oil-loving (hydrophilic) portion of the structure and the remaining portion is silicone-soluble (silliphilic). The number of a and b units and the length of the R alkyl group determine the properties of the silicone polymer. For instance, the length of the alkyl chain is the salient factor in determining its melting point of the alkyl dimethicone. In addition, waxes based on alkyl groups of 16 or less carbon atoms are liquid at room temperature, whereas those with 18 or more carbon atoms are solid; the melting point increases as the carbon length goes up (see Table 1).
It is important to note that a material’s INCI name is determined solely by its R group and does not take these a and b values into consideration. The formulation implications of this effect are that polymers having the same INCI name can function differently in a formulation—one imparting a hydrophobic feel and the other a hydrophilic feel. Generally, the lower molecular weight alkyl dimethicone compounds provide the most hydrophilic feel. Further, formulations with exactly the same label can feel different. Therefore, the INCI name often does not assist in the selection of a proper alkyl silicone polymer.
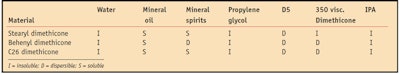
Solubility is another function related to the material’s structure. Liquid alkyl dimethicone can be used as an additive to improve the solubility of polar oils in formulations. Figure 2 depicts the addition of behenyl dimethicone to soybean oil, and Table 2 shows the solubility of alkyl silicones in a variety of solvents.
As noted, alkyl silicones go to the air/oil interface and act as surfactants, lowering the surface tension. The presence of both oil soluble and silicone soluble moieties in the same molecule causes this effect. This reduced surface tension makes oil feel like D5 silicone. As their concentration increases, micelles form. This property makes them amphiphilic and allows them to function at low concentrations. If an alkyl silicone with a melting point above ambient temperature is used it will form a reversible thixotrophic gel that liquefies under pressure and upon heating, and re-solidifies upon cooling. Altering the ratio of alkyl to silicone component in alkyl dimethicone the molecule will change the clarity of the gel and occlusivity of the blend (see Figure 3). Here, behenyl dimethicone was added to the oil with differing amounts of silicone group present.
Multiple Domain Silicones
The alkyl silicone surfactants discussed thus far have consisted of a single alkyl group on a silicone backbone. However, a series of patent-pending alkyl dimethicones have been developed that contain two different alkyl groups—one a liquid at ambient temperatures, and another a solid at ambient temperatures. One such polymer conforms to the structure shown in Figure 4.
The specific structures of the R groups in the solid and liquid domains have a profound effect on the rheology and aesthetics of a formulation and enable variations in characteristics such as cushion and playtime (see Cushion and Playtime). Differences in formulas containing multi-domain versus single domain silicone are clearly visible, as shown in Figure 5.
The two samples shown have exactly the same composition of C22 and C16 except one consists of a blend of two alkyl silicones that have a single alkyl group, the other has the two alkyl groups co-reacted on a single silicone backbone. The presence of the liquid portion of the molecule inhibits formulas from becoming hard solids, instead forming soft, thixotrophic gels that are translucent, that liquefy under pressure, and that have a cushion effect yet a short playtime—meaning the material rubs out rapidly.
Photomicroscopy of the two materials shows that the multi-domain silicone polymer is highly structured while the blend of the two single domain silicone polymers is random and lacks structure (see Figure 6). It is this structure that accounts for the different functionality between the polymers.
In this case, the INCI names also recognize the difference. The blend of the two single domain silicone molecules has the INCI name: Behenyl Dimethicone (and) Acetyl Dimethicone, while the multi-domain silicones have the INCI name: Benehyl/Cetyl Dimethicone.
Characterization
The two samples from Figure 5 were compared by an independent laba that characterized them as being different from one another. Sample 1, the multi-domain silicone, was observed as a translucent gel at room temperature. It flowed under pressure and was composed of two phases: a liquid phase at room temperature, and a solid crystalline phase whose relatively large, elongated crystals scattered light to make the product appear translucent or colorless—as if the crystals were absent. The crystalline phase melted entirely at 38°C but re-crystallized when the temperature dropped below this level.
Sample 2, the blend of two single-domain silicones, was classified as an opaque, waxy, white crystalline solid at room temperature whose color and solid phase structure were attributed to a colorless, interlocking crystal composition that melted at 56°C; this transformation was reported as reversible.
According to the studies, the crystals in sample 1 appeared more like liquid crystals than ordinary crystals, such as those forming the waxy solid in sample 2. The authors note that while this has not yet been confirmed, initial observations point in this direction—the most compelling evidence being the disappearance of birefringent crystals in a thin film of sample 1 when pressure was applied to the coverslip, followed by their reappearance once the pressure was released.
Adjusting Formulation Parameters
The capability of changing a formula’s properties using alkyl silicones was tested in sample sunscreens (see Formulas 1a-c). These formulas were tested for SPF on volunteers using a solar simulator lamp with a continuous light spectrum in the UVA and UVB range (290-400 nm). The spectral output of the solar simulator was calibrated according to the US Food and Drug Administration’s requirements2 and the SPF test for all three formulas was performed on the same subjects.
The average SPF values for the sample formulas were: Formula 1a, SPF 19; Formula 1b, SPF 28 (very water resistant or VWR); and Formula 1c, SPF 29 (VWR). Based on these results, the multi-domain silicone boosted SPF in Formula 1b, compared with a control formula excluding a waterproofing film-former. Use of the properly selected alkyl silicone therefore resulted in a more uniform film of sunscreen on the skin and increased the SPF. Based on the results of this study, multi-domain silicones were found to be equivalent to the well-known waterproofing film-former, VP/eicosene copolymer. These multi-domain polymers and their function in formulas will be a topic of further discussion in a follow-up article.
Conclusions
Many interesting properties are imparted by amphiphilic surfactant silicone compounds. These materials lower surface tension, which can alter properties such as wetting, emulsification, foaming and gellation, depending on their specific structure. Since INCI names do not relate to their structure, formulators will need additional details to construct meaningful models, and since silicone polymers typically are incorporated into formulations, it is critically important for formulators to consider their interactions with the other components in a formula. The further evaluation of the effects of silicone surfactants in formulations could be an ideal topic for a computer-assisted evaluation of numerous formulations.
The ability to use this high definition polymer approach to selecting silicones for personal care products will allow formulators to optimize formulations. The amount of silicone in formulations will decrease as more efficient polymers are selected; for example, silicone fluids and cyclic silicones can be replaced with natural oils when the properly selected alkyl silicones are used. This reduction in the overall concentration of silicone in formulations will result in “greening with silicones.”
References
Send e-mail to [email protected].
- E Rochow, An Introduction–Chemistry of Silicones, John Wiley and Sons: Indianapolis (1947)
- Spectral Output Requirements for Testing Sunscreen Drug Products for Over-the-counter Human Use, Proposed Amendment of Final Monograph, CFR Part 352.70 (b) Light Sources, Federal Register, vol 72, No. 165 (Aug 27, 2007); and the International Sun Protection Factor (SPF) Test Method (May 2006)