The International Cooperation on Cosmetics Regulation (ICCR) and the International Associations Collaboration (IAC) initiated a preservative defense project to address the continued reductions in the number of preservatives and preservative systems used in cosmetics.1 For instance, numerous manufacturers have used methylisothiazolinone (MIT) at its upper limit, which likely caused rapid increases in the number of contact allergies, prompting the European Union to prohibit its use in leave-on products.2–4 Experts within the Japanese Cosmetic Industry Association (JCIA) have also been discussing the challenges of cosmetic preservation.
As such, it is vital to design formulations with maximum microbe protection using minimum preservative quantities. By actively pursuing minimum preservative use, the industry will defend today’s available preservative solutions against more stringent restrictions and worsening reputations. This strategy is thus referred to as an “offensive defense.” In relation, it is important for manufacturers to understand strategies to establish appropriate preservative systems, as well as their aggressive use, and to be knowledgeable about the overarching preservative defense project.
The general properties of preservatives and their uses have been reported.5, 6 However, this paper aims to describe the basic theory and essence of preservative systems, as well as methods for determining appropriate preservative levels in formulations as a primer for the larger offensive defense strategy.
Microbial Control in Cosmetics
Since the middle of the 20th century, cosmetics have been known to be susceptible to contamination by organisms such as Gram-negative bacteria. At that time, eye-area products contaminated by Pseudomonas aeruginosa caused eyesight loss,7–10 which highlighted the necessity to initiate urgent action, and various efforts were subsequently undertaken.
As one major response, the International Organization for Standardization/Technical Committees 217 (ISO/TC217) was initiated in 1997, and discussions on microbial issues began in 2001 with working group 1 (WG1) in a review titled, “Microbiological Standards and Limits.” Eleven total ISO documents have since been published, and experts from various countries have continued the discussion for more than 10 years. Furthermore, since test methods, microbiological limit standards, preservative efficacy standards and risk evaluations have been completed, test methods and standards for microbial control have been established.11
The Need for Preservatives and Oversight
Cosmetics are repeatedly used over long periods and therefore differ from food, which is typically consumed before it decays. For this reason, preservatives are added to cosmetics to prevent microbial growth and ensure product quality and consumer safety; except for cosmetics that are inherently microbiocidal or microbiostatic.
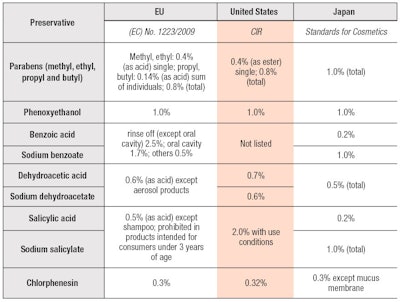
However, since preservatives kill microorganisms or prevent their growth, they can also affect the human skin. As such, many countries regulate preservative types and limit their quantities to avoid safety issues caused by their excessive use. For instance, Europe has Cosmetics Regulation 1223/2009 Annex V,12 the United States has a positive Cosmetic Ingredient Review (CIR) list13 and Japan has a positive list for preservatives in Standards for Cosmetics (see Table 1).14
Effective Preservation
As stated, countries regulate preservative types and upper limits. However, these regulations do not necessarily ensure their safe use. The rapid increase in cases associated with MIT sensitization provides an example.3
EU cosmetic regulations had permitted MIT at 0.01% in leave-on and rinse-off products, and 100-ppm MIT formulations were easy and affordable in many countries. Increasing numbers of manufacturers used it at this upper limit, which is probably what caused the increase in contact allergies. Subsequently, the EU was prompted to prohibit the use of MIT in leave-on products and limit its use in rinse-off products to 0.0015%.
As Figure 1 illustrates, no ideal, safe and effective preservative suitable for cosmetics or effective against all microorganisms has yet been developed. Therefore, to control microorganisms that deteriorate the quality of cosmetics, it is important to understand preservative characteristics in given formulations, and to give an indication of appropriate types and formulation volumes, rather than establishing upper limits for one preservative.
In addition, recently, preservative alternatives such as caprylyl glycol, ethylhexylglycerin and hexylene glycol have demonstrated efficacy at low concentrations and are being used more frequently. Preservative boosters such as alcohol and dihydric glycol are also used with preservatives. As such, safer preservative systems can be developed since combining these materials reduces the amount of actual preservatives required.
Since preservatives kill microorganisms or prevent their growth, they can also affect human skin. As such, many have usage limits.
Preservation in Practice
Not all cosmetic products require preservatives with similar efficacies, and the choice will depend on factors such as product category, use, container, volume and sales region. In addition, the effect of preservatives or antimicrobial compounds can vary in different formulations; e.g., some raw materials such as ester oils can reduce their effectiveness. As described, the safety of preservative systems should also be critically considered.
In addition, note that since preservative systems differ among companies, it is difficult to draw general conclusions. However, knowledge of the basic properties of preservatives and antimicrobial compounds, and their usage requirements, must be shared among cosmetic chemists. Reference information on frequently used cosmetic preservatives is thus provided here.
Preservatives
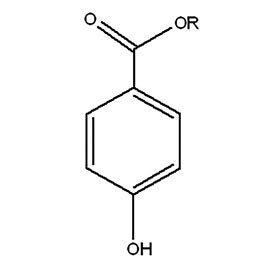
Parabens
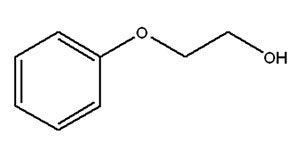
Phenoxyethanol
 Attributes include:
Attributes include:- Useful in various products due to its water solubility;
- Effective at wide pH ranges;
- Less effective than parabens, especially for mold;
- Volatile material, exercise caution; and
- Carries some odor.
Acid-based Preservatives
Attributes include:
- Effective under acidic conditions but weakened at neutral pH ranges since the undissociated form is active (see Figure 2); and
- Effective pH range differs according to product type.
Benzoic Acid and its Sodium Salt
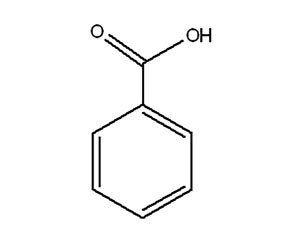 Attributes include:
Attributes include:- Effective under acidic conditions and loses effectiveness at neutral pH;
- Effects are reduced by complex formation with cationic components;
- Often used for relatively acidic shampoos.
Dehydroacetic Acid and its Sodium Salt
 Attributes include:
Attributes include:- Effective under acidic conditions and at neutral pH;
- Often used for mascara and eyeliners due to low adsorption to film-forming materials and brushes;
- Effects are reduced by complex formation with cationic components; and
- May cause discoloration due to reaction with metal salts.
Salicylic Acid and its Sodium Salt
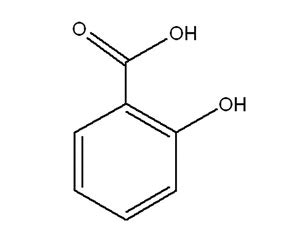 Attributes include:
Attributes include:- Effective under acid conditions and less effective than benzoic acid at neutral pH;
- Effects are reduced by complex formation with cationic components;
- Used as a stratum corneum softener and for acne control; and
- Legally permitted upper limits of acids and salts differ in some countries.
Quaternary Ammonium Salts
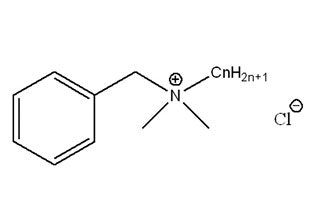 Attributes include:
Attributes include:- Effective at wide pH ranges;
- Strong effects at low concentrations; benzalkonium chloride is effective at < 100 ppm;
- Effects are reduced when combined with anions due to cationic properties; and
- Must be combined with other preservatives since some microorganisms can adapt to it and develop resistance.
Chlorphenesin
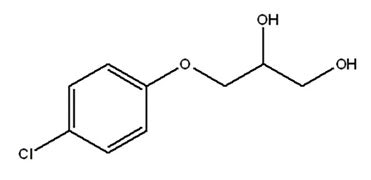 Attributes include:
Attributes include:- Used for water-based products, powders and solid products—e.g., makeup powders and eye shadows—due to broad-spectrum antibacterial activity, similar to paraben; and
- Adsorption to nylon and other fabrics is less than paraben.
MIT/Methylchloroisothiazolinone Mixture
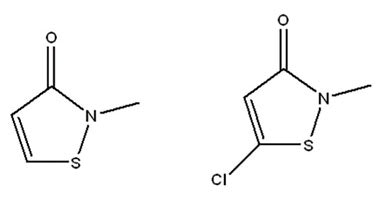 Attributes include:
Attributes include:- Highly effective at low concentrations (on the order of just a few ppm);
- Mainly utilized for in-bath formulations having the potential for water immersion during use;
- Unstable in alkaline pH and degrades at high temperatures; and
- Avoid its isolated use in formulations as microorganisms easily adapt to and resist it.
Alcohol Compounds
Ethanol, Isopropyl Alcohol
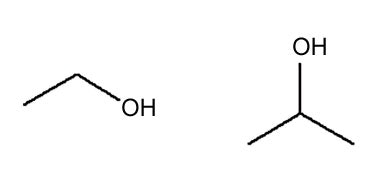 Attributes include:
Attributes include:- Considered low risk (ISO 29621)16 at ≥ 20%;
- Surfactants increase its effects;
- Bacterial resistance does not readily develop; and
- Volatile in nature; exercise caution with temperature and packaging.
Benzyl Alcohol
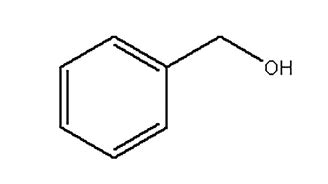
Attributes include:
- High versatility with aqueous products due to its relatively high water solubility;
- Effective against various microorganisms at a wide pH range;
- Peculiar smell can be unpleasant; and
- Categorized as a preservative in the EU.
Diol Compounds
1,2-Pentanediol, 1,2-Hexanediol, Caprylyl Glycol(1,2-Octanediol)
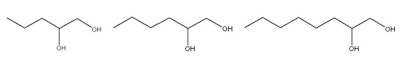
Attributes include:
- Act as moisturizers with antimicrobial effects;
- Often used for paraben-free and preservative-free product claims since legally, they are not preservatives;
- Longer chain diols have stronger effects than shorter chains; e.g., caprylyl glycol is effective at < 1% and 1,2-pentanediol is generally used at 1–5%; and
- Esther oils reduce the effects of caprylyl glycol due to its low aqueous solubility.
1,3-Butylene Glycol, 1,3-Propanediol, Dipropylene Glycol
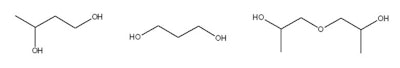 Attributes include:
Attributes include:- Combinations of preservatives with high percentages of these glycols have synergistic effects and can reduce the amount of preservatives required (see Figure 3);15 and
- Can prevent the development of resistant bacteria.
Added Defenses
Ethylhexylglycerin
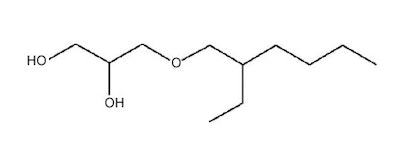 Attributes include:
Attributes include:- Invented as an antimicrobial agent that prevents osmidrosis; and
- Effective at < 1% depending on the formulation; e.g., ester oils reduce its effectiveness.
Chelating Agents
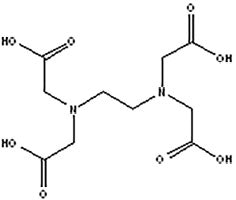 For example, ethylenediaminetetraacetic acid (EDTA); attributes include:
For example, ethylenediaminetetraacetic acid (EDTA); attributes include:- When used individually, effective against Gram-negative bacteria, as they chelate metal ions from microbe membranes;
- May inhibit microbial resistance, adaptation or both to preservatives when combined with preservatives and/or other antimicrobial compounds; and
- Effective concentration range depends on the formulation.
Factors Affecting Preservative Efficacy
While some products have properties that enable their use without the addition of antimicrobial compounds, as described in ISO 29621,16 many do not. Therefore, to ensure the success of the offensive defense strategy, it is important to consider several additional factors and interactions that can impact preservation in given formulations.
pH: The approximately optimal pH levels for microbe growth are as follows: for bacteria, pH 6–8; for mold, pH 4–6; and for yeast, pH 5–7. Preservative requirements will be lower in pH ranges other than these, which are not optimal for microbe growth. To ensure low risk for pH effects, ISO 29621 would be helpful (see 5-4).16
Water activity (Aw): Water activity refers to the amount of water used by microorganisms, not the water content. If the Aw is low, < 0.75, the risk of microbial growth is low (see ISO 29621).16 However, a low Aw does not actively kill microorganisms. Therefore, preventing microbial contamination during manufacturing and optimizing preservative formulation designs are important. Note that it is also necessary to prevent increased Aw for water-absorbent products.
Extracts: Some extracts have added antimicrobial effects. However, other extract components (e.g., amino acids, disaccharides and minerals) may increase microbial tolerance. In the latter case, it is necessary to establish stronger preservation efficacy by combining preservatives, preservative alternatives, preservative boosters or any combination of these.
Fragrances: Consider that many fragrances also have antimicrobial effects.
Powdered materials: Some powders, such as zinc oxide and flowers of zinc, also have antimicrobial effects. In contrast, some powdered materials, such as nylon, tend to adsorb preservatives and inhibit their effectiveness. When the latter materials are used, preservatives that are not adsorbed should be used, or preservative system levels should be increased to compensate for the expected adsorption.
Oil/water distribution: Also be sure to consider oil/water distribution, to ensure effective preservative use. For example, long-chain parabens are difficult to dissolve in a water phase and lose their efficacy when considerable amounts of ester oils, UV absorbers or both are used.
Package, Applicator and Use Considerations
Also crucial to the offensive defense strategy is choosing packaging that can prevent and reduce contamination. Dispensers, pumps and one-way discharge tubes can prevent contamination during use. In addition, single use packaging is effective for preventing contamination. When using such applicators and packaging, it is possible to reduce the need for preservatives.
In contrast, preservative efficacy is reduced by paraben adsorption on nylon brushes and nitrile rubber. Preservative deactivation or decomposition also may occur due to UV exposure, depending on the transparency of the container material. Thus, the packaging and applicator should be composed of material that does not bind, deactivate or decompose preservatives.
For an offensive defense strategy, the cooperation of formulators and microbiologists is essential.
Refillable products also pose a higher risk of microbial contamination than those intended for one-time use. If products are to be refilled, it is important to provide consumers with clear instructions; for example, washed containers should be dried well.
Finally, the mode of use is an important variable. For instance, products used in-bath are easily contaminated by water, which may reduce preservative efficacy. This risk must be considered.
Furthermore, the use environment can impact preservative efficacy. Caution must be exercised, for example, with sheet and tube products due to the volatility of preservative components such as alcohol and phenoxyethanol in hot and humid environmental conditions. Once again, close attention should be paid to potential microbial growth caused by moisture uptake by low-Aw products.
Summary
Preservative systems depend on the formulation design; therefore, the efficacies of individual formulations differ. The various points described here must be considered, or the formulation efficacy will not meet expectations. Failure to apply this knowledge will cause the excessive use of preservatives, leading to the utilization of maximum quantities; which as described, is an undesired scenario.
It is critical for researchers to acquire this fundamental knowledge and these technical skills. Applying this knowledge will enhance the efficacy of the accumulated data and the proficiency of each manufacturer, and thereby ensure safe and effective formulas. Therefore, the cooperation of formulators and microbiologists is essential. This will elevate the level of preservative system design and maintain the diversity of the preservative palette.
This activity by researchers may appear defensive in nature, but it will work offensively and proactively toward a more successful future. How? This offensive defense strategy will help to maintain the preservative and preservative booster palette.
References
- https://ec.europa.eu/docsroom/documents/17203/attachments/1/translations/en/renditions/native (Accessed Feb 2, 2018)
- R Urwin, K Warburton, M Carder, S Turner, R Agius and SM Wilkinson, Methylchloroisothiazolinone and methylisothiazolinone contact allergy: An occupational perspective, Contact Dermatitis 72(6) 381-386 (2015) doi: 10.1111/cod.12379
- MD Lundov, C Zachariae and JD Johansen, Methylisothiazolinone contact allergy and dose-response relationships, Contact Dermatitis 64(6) 330-336 (2011) doi: 10.1111/j.1600-0536.2011.01901.x
- http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2016.198.01.0010.01.ENG&toc=OJ:L:2016:198:TOC (Accessed Aug 1, 2017)
- ES Abrutyn, Optimizing formula preservation, Cosm & Toil 125(3) 22-28 (2010)
- SP Denyer and RM Baird, Antimicrobial preservatives and their properties, Guide to Microbiological Control in Pharmaceuticals and Medical Devices, second edition, CRC Press, Boca Raton, FL USA 324-344 (2006)
- C Ishizeki and H Kurata, Problem on the microbiological control for cosmetics, Frag J 41 12-18 (1980)
- WH Spencer, Pseudomonas aeruginosa infections of the eye, Calif Med 79(6) 438-443 (1953)
- FR Reid and TO Wood, Pseudomonas corneal ulcer. The causative role of contaminated eye cosmetics, Arch Ophthalmol 97(9) 1640-1641 (1979) doi:10.1001/archopht.1979.01020020208002
- LA Wilson, JW Kuehne, SW Hall and DG Ahearn, Microbial contamination in ocular cosmetics, Am J Ophthalmol 71(6) 1298-1302 (1971)
- https://www.iso.org/standards.html (Accessed Jul 1, 2017)
- https://data.europa.eu/euodp/en/data/dataset/cosmetic-ingredient-database-list-of-preservatives-allowed-in-cosmetic-products (Accessed Nov 1, 2016)
- http://www.cir-safety.org/ingredients (Accessed Nov 1, 2016)
- http://www.mhlw.go.jp/file/06-Seisakujouhou-11120000-Iyakushokuhinkyoku/0000032704.pdf (Accessed Nov 1, 2016)
- M Ookawa, et al., in progress
- ISO 29621:2010, Cosmetics—Microbiology—Guidelines for the risk assessment and identification of microbiologically low-risk products, available at iso.org/standard/45592.html (Accessed Jan 29, 2018)