Personal care formulators are aware that “natural” products can be difficult to preserve…but not always for the reasons they might think. Certainly, natural thickeners (like xanthan) and plant extracts can carry their own bioburdens, increasing the workload on the preservative in the formulation. However, the higher potential for natural materials to carry organisms into the formulation process is not the only preservation issue.
In continuing effort to provide customers with the best formulation stewardship, schülke has researched why some formulations are harder to preserve than others. This research led to an interesting finding: natural oils make a “natural” formula more difficult to preserve.
Natural oils and other esterified emollients have replaced traditional mineral oil in many modern personal care products. These newer oil-phase materials are often more polar than traditional oil-phase materials. Traditional preservatives, like formaldehyde-donors and isothiazolinones, are highly water-soluble and remain in the water phase of emulsions, where microorganisms exist. However, many newer alternative preservative systems (i.e., containing caprylyl glycol, phenethyl alcohol, ethylhexylglycerin) have limited water solubility. Therefore, the partition coefficient between the oil and water phases of preservatives/antimicrobial boosters is important to the role they play in the efficacy of emulsions.
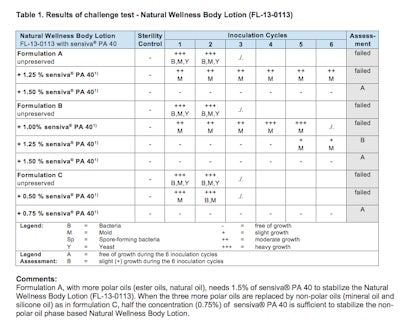
schülke carried out tests varying the composition and polarity of the oil phase in emulsions. Figure 1 shows and oil-in-water lotion formula. Overall oil phase levels remained relatively constant, but the polarity of the oil phases decreased from highly polar in variant A, to highly non-polar in variant C. While the most nonpolar variant (variant C) was easily stabilized using 0.75% sensiva® PA 40, higher levels of sensiva® PA 40 consistent with the increase in polarity of the oil phase were required (Table 1).
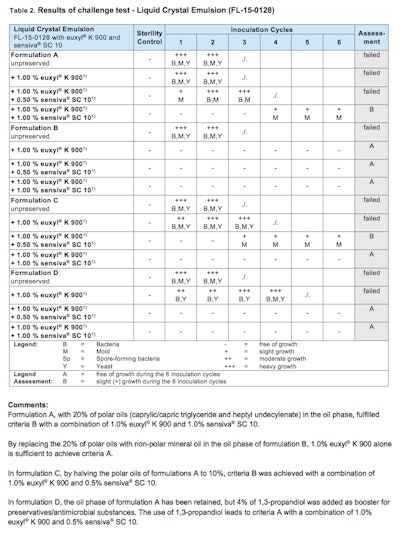
Additionally, a liquid crystal emulsion formula was prepared (see Figure 2). Variant A contained a highly polar oil phase. The polarity of the oil phase was reduced in variant B. The more polar oils were reduced by half in variant C, reducing the overall oil phase. Variant D retained the variant A polar oil phase, but 4.00% propanediol was added as a booster for the preservation system. The results (Table 2) showed variant B (non-polar) was relatively easy to preserve using euxyl® K 900. The lower polar oil phase variant (variant C) required the addition of a preservative booster (sensiva® SC 10) to improve preservative performance. The most highly polar variants (variants A and D) required a higher level of sensiva® SC 10 or a combination of sensiva® SC 10 and propanediol to attain adequate preservation.
The ongoing trend toward “natural” is making the preservation of personal care products more difficult for a variety of reasons. The accompanying trend toward softer, non-traditional preservation systems can add to the complexity of formulating these systems. The preservation experts at schülke understand the changing needs of the personal care industry.
Let schülke help you solve the mysteries of better “natural” formulations.
Disclaimer:
The above paid-for content was produced by and posted on behalf of the Sponsor. Content provided is generated solely by the Sponsor or its affiliates, and it is the Sponsor’s responsibility for the accuracy, completeness and validity of all information included. Cosmetics & Toiletries takes steps to ensure that you will not confuse sponsored content with content produced by Cosmetics & Toiletries and governed by its editorial policy.